A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states
- PMID: 35953545
- PMCID: PMC9448678
- DOI: 10.1038/s41593-022-01131-4
A CRISPRi/a platform in human iPSC-derived microglia uncovers regulators of disease states
Abstract
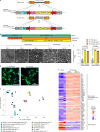
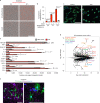
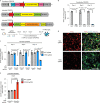
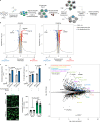
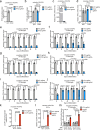
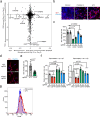
Microglia are emerging as key drivers of neurological diseases. However, we lack a systematic understanding of the underlying mechanisms. Here, we present a screening platform to systematically elucidate functional consequences of genetic perturbations in human induced pluripotent stem cell-derived microglia. We developed an efficient 8-day protocol for the generation of microglia-like cells based on the inducible expression of six transcription factors. We established inducible CRISPR interference and activation in this system and conducted three screens targeting the 'druggable genome'. These screens uncovered genes controlling microglia survival, activation and phagocytosis, including neurodegeneration-associated genes. A screen with single-cell RNA sequencing as the readout revealed that these microglia adopt a spectrum of states mirroring those observed in human brains and identified regulators of these states. A disease-associated state characterized by osteopontin (SPP1) expression was selectively depleted by colony-stimulating factor-1 (CSF1R) inhibition. Thus, our platform can systematically uncover regulators of microglial states, enabling their functional characterization and therapeutic targeting.
© 2022. The Author(s).
Conflict of interest statement
M.A.N. consults for Neuron23. M.A.N. and F.F. participated in this work in part due to a competitively awarded consulting contract between Data Tecnica International, LLC and the National Institutes of Health (USA). J.I. is a cofounder of AcuraStem, Inc. and Modulo Bio, and serves on the scientific advisory board of Spinogenix. L.G. is a founder of Aeton Therapeutics. M.K. is an inventor on US patent 11,254,933 related to CRISPRi and CRISPRa screening; serves on the scientific advisory boards of Engine Biosciences, Casma Therapeutics, Cajal Neuroscience and Alector; and is a consultant to Modulo Bio and Recursion Therapeutics. The remaining authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

