Every islet matters: improving the impact of human islet research
- PMID: 35953581
- PMCID: PMC11135339
- DOI: 10.1038/s42255-022-00607-8
Every islet matters: improving the impact of human islet research
Erratum in
-
Author Correction: Every islet matters: improving the impact of human islet research.Nat Metab. 2024 Jul;6(7):1415. doi: 10.1038/s42255-024-01091-y. Nat Metab. 2024. PMID: 38956323 No abstract available.
Abstract
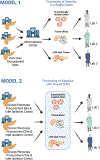
Detailed characterization of human pancreatic islets is key to elucidating the pathophysiology of all forms of diabetes, especially type 2 diabetes. However, access to human pancreatic islets is limited. Pancreatic tissue for islet retrieval can be obtained from brain-dead organ donors or from individuals undergoing pancreatectomy, often referred to as 'living donors'. Different protocols for human islet procurement can substantially impact islet function. This variability, coupled with heterogeneity between individuals and islets, results in analytical challenges to separate genuine disease pathology or differences between human donors from experimental noise. There are currently no international guidelines for human donor phenotyping, islet procurement and functional characterization. This lack of standardization means that substantial investments from multiple international efforts towards improved understanding of diabetes pathology cannot be fully leveraged. In this Perspective, we overview the status of the field of human islet research, highlight the challenges and propose actions that could accelerate research progress and increase understanding of type 2 diabetes to slow its pandemic spreading.
© 2022. Springer Nature Limited.
Conflict of interest statement
Conflicts of Interest
ALG and ACP are members of the Human Pancreas Atlas Program (HPAP), ALG is a member of the Integrated Islet Distribution Program. ALG and P.M are members of the Horizon 2020 funded T2DSystems. ALG, ACP, Ma.Sa are members of the Human Islet Research Network. P.M. M.I. and Mi.So. are members of Rhapsody and IMIDIA. Through their membership of these consortia the authors have received financial support as outlined in the acknowledgements.
Figures



References
Publication types
MeSH terms
Grants and funding
- U01 DK085545/DK/NIDDK NIH HHS/United States
- MR/V011979/1/MRC_/Medical Research Council/United Kingdom
- U01 DK120429/DK/NIDDK NIH HHS/United States
- R01 DK068471/DK/NIDDK NIH HHS/United States
- UC4 DK112217/DK/NIDDK NIH HHS/United States
- R24 DK106755/DK/NIDDK NIH HHS/United States
- U01 DK123716/DK/NIDDK NIH HHS/United States
- U01 HG012059/HG/NHGRI NIH HHS/United States
- R01 DK114427/DK/NIDDK NIH HHS/United States
- UH3 DK122639/DK/NIDDK NIH HHS/United States
- U24 DK138512/DK/NIDDK NIH HHS/United States
- 200837 /WT_/Wellcome Trust/United Kingdom
- U01 DK112217/DK/NIDDK NIH HHS/United States
- U24 DK098085/DK/NIDDK NIH HHS/United States
- R01 DK078803/DK/NIDDK NIH HHS/United States
- R01 DK122607/DK/NIDDK NIH HHS/United States
- U01 DK105535/DK/NIDDK NIH HHS/United States
- UC4 DK112232/DK/NIDDK NIH HHS/United States
- I01 BX000666/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- U01 DK123743/DK/NIDDK NIH HHS/United States
- P30 DK116074/DK/NIDDK NIH HHS/United States
- UM1 DK126185/DK/NIDDK NIH HHS/United States
- 200837/Z/16/Z/WT_/Wellcome Trust/United Kingdom

