3D Paper-based milk adulteration detection device
- PMID: 35953582
- PMCID: PMC9372070
- DOI: 10.1038/s41598-022-17851-3
3D Paper-based milk adulteration detection device
Abstract
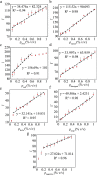
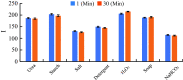
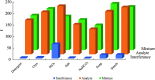
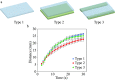
Milk adulteration is a common problem in developing countries, and it can lead to fatal diseases in humans. Despite several studies to identify different adulterants in milk samples, the effects of multiple adulterants remain unexplored. In this work, a three-dimensional (3D) paper-based microfluidic device is designed and fabricated to simultaneously detect multiple chemical adulterants in milk. This device comprises a top cover, a bottom cover, and a middle layer composed of transportation and a detection zone. By making cuts on the middle layer's support, the device's flow path is characterised by optimum and uniform velocity. For the first time, seven adulterants (urea, detergents, soap, starch, hydrogen peroxide, sodium-hydrogen-carbonate, and salt) are detected in the milk sample simultaneously with specificity evaluation and detailed color interference analysis. Only 1-2 mL of sample volume is required to detect 7 adulterants at one time. We have used only 10 [Formula: see text]L of the reagent's volume for the colorimetric reaction and found the results within a few seconds. Observation reveals that the limit of detection (LOD) of the adulterants lies in the range between [Formula: see text] (vol./vol.) to [Formula: see text] (vol./vol.) using the colorimetric detection technique. The unknown quantity of the added adulterants is measured using the calibration curves obtained from the experiments results. The repeatability and reproducibility of the process, sensitivity, and the linear range of detection of the calibration curves and the statistical study of the color intensity data are thoroughly analysed herein. In any resource-limited setting, this simple, portable, and user-friendly 3D microfluidic device is expected to be used for testing liquid foods before consumption.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Trivedi U, et al. Potentiometric biosensor for urea determination in milk. Sens. Actuators B Chem. 2009;140:260–266. doi: 10.1016/j.snb.2009.04.022. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

