Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts
- PMID: 35953672
- PMCID: PMC9849136
- DOI: 10.1038/s41587-022-01408-w
Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts
Abstract
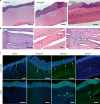
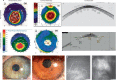
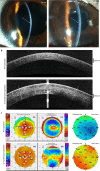
Visual impairment from corneal stromal disease affects millions worldwide. We describe a cell-free engineered corneal tissue, bioengineered porcine construct, double crosslinked (BPCDX) and a minimally invasive surgical method for its implantation. In a pilot feasibility study in India and Iran (clinicaltrials.gov no. NCT04653922 ), we implanted BPCDX in 20 advanced keratoconus subjects to reshape the native corneal stroma without removing existing tissue or using sutures. During 24 months of follow-up, no adverse event was observed. We document improvements in corneal thickness (mean increase of 209 ± 18 µm in India, 285 ± 99 µm in Iran), maximum keratometry (mean decrease of 13.9 ± 7.9 D in India and 11.2 ± 8.9 D in Iran) and visual acuity (to a mean contact-lens-corrected acuity of 20/26 in India and spectacle-corrected acuity of 20/58 in Iran). Fourteen of 14 initially blind subjects had a final mean best-corrected vision (spectacle or contact lens) of 20/36 and restored tolerance to contact lens wear. This work demonstrates restoration of vision using an approach that is potentially equally effective, safer, simpler and more broadly available than donor cornea transplantation.
© 2022. The Author(s).
Conflict of interest statement
M.R. holds stock and relevant patents in LinkoCare Life Sciences AB, a spin-off company from Linköping University developing BPCDX and related products. He also serves on the board of directors of the company. The terms of his arrangements have been approved by Linköping University in accordance with its policy on objectivity in research. S.T. is business development manager of LinkoCare and serves on the board of directors of LinkoCare Life Sciences AB, as an unpaid board member. N.L., M.J. and N.S. serve on the scientific advisory board of LinkoCare Life Sciences AB, as unpaid advisory board members. Other co-authors have no competing interests.
Figures



Comment in
-
Accessible bioengineered corneal tissue to address a blinding disease globally.Nat Biotechnol. 2023 Jan;41(1):25-26. doi: 10.1038/s41587-022-01409-9. Nat Biotechnol. 2023. PMID: 35953674 No abstract available.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

