Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease
- PMID: 35953717
- PMCID: PMC9499867
- DOI: 10.1038/s41591-022-01925-w
Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease
Erratum in
-
Publisher Correction: Plasma p-tau231 and p-tau217 as state markers of amyloid-β pathology in preclinical Alzheimer's disease.Nat Med. 2022 Sep;28(9):1965. doi: 10.1038/s41591-022-02037-1. Nat Med. 2022. PMID: 36100683 Free PMC article. No abstract available.
Abstract
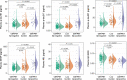
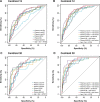
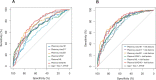
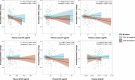
Blood biomarkers indicating elevated amyloid-β (Aβ) pathology in preclinical Alzheimer's disease are needed to facilitate the initial screening process of participants in disease-modifying trials. Previous biofluid data suggest that phosphorylated tau231 (p-tau231) could indicate incipient Aβ pathology, but a comprehensive comparison with other putative blood biomarkers is lacking. In the ALFA+ cohort, all tested plasma biomarkers (p-tau181, p-tau217, p-tau231, GFAP, NfL and Aβ42/40) were significantly changed in preclinical Alzheimer's disease. However, plasma p-tau231 reached abnormal levels with the lowest Aβ burden. Plasma p-tau231 and p-tau217 had the strongest association with Aβ positron emission tomography (PET) retention in early accumulating regions and associated with longitudinal increases in Aβ PET uptake in individuals without overt Aβ pathology at baseline. In summary, plasma p-tau231 and p-tau217 better capture the earliest cerebral Aβ changes, before overt Aβ plaque pathology is present, and are promising blood biomarkers to enrich a preclinical population for Alzheimer's disease clinical trials.
© 2022. The Author(s).
Conflict of interest statement
E.V. is a co-founder of ADx NeuroSciences. T.A.D. is an employee and shareholder of Eli Lilly and Company. J.L.M. is currently a full-time employee of Lundbeck and has previously served as a consultant or on advisory boards for the following for-profit companies, or has given lectures in symposia sponsored by the following for-profit companies: Roche Diagnostics, Genentech, Novartis, Lundbeck, Oryzon, Biogen, Lilly, Janssen, Green Valley, MSD, Eisai, Alector, BioCross, GE Healthcare and ProMIS Neurosciences. J.L.D. has served as a consultant for Genotix Biotechnologies Inc., Gates Ventures, Karuna Therapeutics, AlzPath Inc. and Cognito Therapeutics, Inc. J.L.D. received research support from ADx Neurosciences, Roche Diagnostics and Eli Lilly and Company in the past 2 years. H.Z. has served on scientific advisory boards for Eisai, Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, Nervgen, AZTherapies and CogRx, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS). J.D.G. has received speaker’s fees from Philips and Biogen and research support from GE Healthcare, Roche and Roche Diagnostics. M.S.C. has served as a consultant and on advisory boards for Roche Diagnostics International Ltd, and has given lectures in symposia sponsored by Roche Diagnostics, S.L.U. and Roche Farma, S.A. K.B. has served as a consultant, on advisory boards or at data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Ono Pharma, Roche Diagnostics and Siemens Healthineers, and is a co-founder of BBS in Gothenburg. The remaining authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

