Translationally controlled tumor protein: the mediator promoting cancer invasion and migration and its potential clinical prospects
- PMID: 35953758
- PMCID: PMC9381325
- DOI: 10.1631/jzus.B2100910
Translationally controlled tumor protein: the mediator promoting cancer invasion and migration and its potential clinical prospects
Abstract
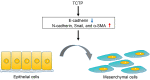
Translationally controlled tumor protein (TCTP) is a highly conserved multifunctional protein localized in the cytoplasm and nucleus of eukaryotic cells. It is secreted through exosomes and its degradation is associated with the ubiquitin-proteasome system (UPS), heat shock protein 27 (Hsp27), and chaperone-mediated autophagy (CMA). Its structure contains three α-helices and eleven β-strands, and features a helical hairpin as its hallmark. TCTP shows a remarkable similarity to the methionine-R-sulfoxide reductase B (MsrB) and mammalian suppressor of Sec4 (Mss4/Dss4) protein families, which exerts guanine nucleotide exchange factor (GEF) activity on small guanosine triphosphatase (GTPase) proteins, suggesting that some functions of TCTP may at least depend on its GEF action. Indeed, TCTP exerts GEF activity on Ras homolog enriched in brain (Rheb) to boost the growth and proliferation of Drosophila cells. TCTP also enhances the expression of cell division control protein 42 homolog (Cdc42) to promote cancer cell invasion and migration. Moreover, TCTP regulates cytoskeleton organization by interacting with actin microfilament (MF) and microtubule (MT) proteins and inducing the epithelial-mesenchymal transition (EMT) process. In essence, TCTP promotes cancer cell movement. It is usually highly expressed in cancerous tissues and thus reduces patient survival; meanwhile, drugs can target TCTP to reduce this effect. In this review, we summarize the mechanisms of TCTP in promoting cancer invasion and migration, and describe the current inhibitory strategy to target TCTP in cancerous diseases.
Keywords: Cancer; Clinical drugs; Invasion; Migration; Translationally controlled tumor protein (TCTP).
Figures
Similar articles
-
Elusive Role of TCTP Protein and mRNA in Cell Cycle and Cytoskeleton Regulation.Results Probl Cell Differ. 2017;64:217-225. doi: 10.1007/978-3-319-67591-6_11. Results Probl Cell Differ. 2017. PMID: 29149411 Review.
-
Translationally controlled tumor protein induces epithelial to mesenchymal transition and promotes cell migration, invasion and metastasis.Sci Rep. 2015 Jan 27;5:8061. doi: 10.1038/srep08061. Sci Rep. 2015. PMID: 25622969 Free PMC article.
-
Acetylation of translationally controlled tumor protein promotes its degradation through chaperone-mediated autophagy.Eur J Cell Biol. 2017 Mar;96(2):83-98. doi: 10.1016/j.ejcb.2016.12.002. Epub 2017 Jan 17. Eur J Cell Biol. 2017. PMID: 28110910
-
Dihydroartemisinin inhibits TCTP-dependent metastasis in gallbladder cancer.J Exp Clin Cancer Res. 2017 May 15;36(1):68. doi: 10.1186/s13046-017-0531-3. J Exp Clin Cancer Res. 2017. PMID: 28506239 Free PMC article.
-
To cease or to proliferate: new insights into TCTP function from a Drosophila study.Cell Adh Migr. 2007 Jul-Sep;1(3):129-30. doi: 10.4161/cam.1.3.4901. Epub 2007 Jul 16. Cell Adh Migr. 2007. PMID: 19262129 Free PMC article. Review.
Cited by
-
Immune inflammatory regulation in Anti-NMDAR encephalitis: insights from transcriptome analysis.Front Neurol. 2025 May 9;16:1568274. doi: 10.3389/fneur.2025.1568274. eCollection 2025. Front Neurol. 2025. PMID: 40417115 Free PMC article.
-
The C. elegans anchor cell: A model to elucidate mechanisms underlying invasion through basement membrane.Semin Cell Dev Biol. 2024 Feb 15;154(Pt A):23-34. doi: 10.1016/j.semcdb.2023.07.002. Epub 2023 Jul 6. Semin Cell Dev Biol. 2024. PMID: 37422376 Free PMC article. Review.
-
Rhodosporidium toruloides-a new surrogate model to study rapamycin induced effects on human aging and cancer.Cell Mol Life Sci. 2025 Apr 9;82(1):153. doi: 10.1007/s00018-025-05662-4. Cell Mol Life Sci. 2025. PMID: 40205123 Free PMC article.
-
Mechanistic insights into heat shock protein 27, a potential therapeutic target for cardiovascular diseases.Front Cardiovasc Med. 2023 May 12;10:1195464. doi: 10.3389/fcvm.2023.1195464. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37252119 Free PMC article. Review.
-
The Caenorhabditis elegans anchor cell transcriptome: ribosome biogenesis drives cell invasion through basement membrane.Development. 2023 May 1;150(9):dev201570. doi: 10.1242/dev.201570. Epub 2023 May 4. Development. 2023. PMID: 37039075 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

