Ryanodine receptor 1-mediated Ca2+ signaling and mitochondrial reprogramming modulate uterine serous cancer malignant phenotypes
- PMID: 35953818
- PMCID: PMC9373370
- DOI: 10.1186/s13046-022-02419-w
Ryanodine receptor 1-mediated Ca2+ signaling and mitochondrial reprogramming modulate uterine serous cancer malignant phenotypes
Abstract
Background: Uterine serous cancer (USC) is the most common non-endometrioid subtype of uterine cancer, and is also the most aggressive. Most patients will die of progressively chemotherapy-resistant disease, and the development of new therapies that can target USC remains a major unmet clinical need. This study sought to determine the molecular mechanism by which a novel unfavorable prognostic biomarker ryanodine receptor 1 (RYR1) identified in advanced USC confers their malignant phenotypes, and demonstrated the efficacy of targeting RYR1 by repositioned FDA-approved compounds in USC treatment.
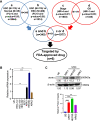
Methods: TCGA USC dataset was analyzed to identify top genes that are associated with patient survival or disease stage, and can be targeted by FDA-approved compounds. The top gene RYR1 was selected and the functional role of RYR1 in USC progression was determined by silencing and over-expressing RYR1 in USC cells in vitro and in vivo. The molecular mechanism and signaling networks associated with the functional role of RYR1 in USC progression were determined by reverse phase protein arrays (RPPA), Western blot, and transcriptomic profiling analyses. The efficacy of the repositioned compound dantrolene on USC progression was determined using both in vitro and in vivo models.
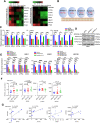
Results: High expression level of RYR1 in the tumors is associated with advanced stage of the disease. Inhibition of RYR1 suppressed proliferation, migration and enhanced apoptosis through Ca2+-dependent activation of AKT/CREB/PGC-1α and AKT/HK1/2 signaling pathways, which modulate mitochondrial bioenergetics properties, including oxidative phosphorylation, ATP production, mitochondrial membrane potential, ROS production and TCA metabolites, and glycolytic activities in USC cells. Repositioned compound dantrolene suppressed USC progression and survival in mouse models.
Conclusions: These findings provided insight into the mechanism by which RYR1 modulates the malignant phenotypes of USC and could aid in the development of dantrolene as a repurposed therapeutic agent for the treatment of USC to improve patient survival.
Keywords: AKT/CREB/PGC-1α signaling pathway and AKT/HK1/2 signaling pathway; RYR1; USC.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Wallbillich JJ, Morris RT, Ali-Fehmi R. Comparing mutation frequencies for homologous recombination genes in uterine serous and high-grade serous ovarian carcinomas: a case for homologous recombination deficiency testing in uterine serous carcinoma. Gynecol Oncol. 2020;159(2):381-386. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

