Limited evidence for blood eQTLs in human sexual dimorphism
- PMID: 35953856
- PMCID: PMC9373355
- DOI: 10.1186/s13073-022-01088-w
Limited evidence for blood eQTLs in human sexual dimorphism
Abstract
Background: The genetic underpinning of sexual dimorphism is very poorly understood. The prevalence of many diseases differs between men and women, which could be in part caused by sex-specific genetic effects. Nevertheless, only a few published genome-wide association studies (GWAS) were performed separately in each sex. The reported enrichment of expression quantitative trait loci (eQTLs) among GWAS-associated SNPs suggests a potential role of sex-specific eQTLs in the sex-specific genetic mechanism underlying complex traits.
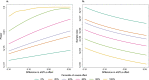
Methods: To explore this scenario, we combined sex-specific whole blood RNA-seq eQTL data from 3447 European individuals included in BIOS Consortium and GWAS data from UK Biobank. Next, to test the presence of sex-biased causal effect of gene expression on complex traits, we performed sex-specific transcriptome-wide Mendelian randomization (TWMR) analyses on the two most sexually dimorphic traits, waist-to-hip ratio (WHR) and testosterone levels. Finally, we performed power analysis to calculate the GWAS sample size needed to observe sex-specific trait associations driven by sex-biased eQTLs.
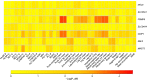
Results: Among 9 million SNP-gene pairs showing sex-combined associations, we found 18 genes with significant sex-biased cis-eQTLs (FDR 5%). Our phenome-wide association study of the 18 top sex-biased eQTLs on >700 traits unraveled that these eQTLs do not systematically translate into detectable sex-biased trait-associations. In addition, we observed that sex-specific causal effects of gene expression on complex traits are not driven by sex-specific eQTLs. Power analyses using real eQTL- and causal-effect sizes showed that millions of samples would be necessary to observe sex-biased trait associations that are fully driven by sex-biased cis-eQTLs. Compensatory effects may further hamper their detection.
Conclusions: Our results suggest that sex-specific eQTLs in whole blood do not translate to detectable sex-specific trait associations of complex diseases, and vice versa that the observed sex-specific trait associations cannot be explained by sex-specific eQTLs.
© 2022. The Author(s).
Conflict of interest statement
T.G.R. is now employed part-time by Novo Nordisk outside of this work. The other authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

