Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer
- PMID: 35953863
- PMCID: PMC9367166
- DOI: 10.1186/s12951-022-01586-4
Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer
Abstract
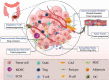
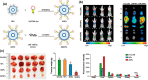
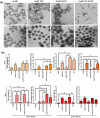
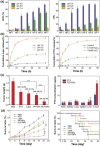
Colorectal cancer is considered one of the major malignancies that threaten the lives and health of people around the world. Patients with CRC are prone to post-operative local recurrence or metastasis, and some patients are advanced at the time of diagnosis and have no chance for complete surgical resection. These factors make chemotherapy an indispensable and important tool in treating CRC. However, the complex composition of the tumor microenvironment and the interaction of cellular and interstitial components constitute a tumor tissue with high cell density, dense extracellular matrix, and high osmotic pressure, inevitably preventing chemotherapeutic drugs from entering and acting on tumor cells. As a result, a novel drug carrier system with targeted nanoparticles has been applied to tumor therapy. It can change the physicochemical properties of drugs, facilitate the crossing of drug molecules through physiological and pathological tissue barriers, and increase the local concentration of nanomedicines at lesion sites. In addition to improving drug efficacy, targeted nanoparticles also reduce side effects, enabling safer and more effective disease diagnosis and treatment and improving bioavailability. In this review, we discuss the mechanisms by which infiltrating cells and other stromal components of the tumor microenvironment comprise barriers to chemotherapy in colorectal cancer. The research and application of targeted nanoparticles in CRC treatment are also classified.
Keywords: Chemotherapeutic resistance; Colorectal cancer; Targeted nanoparticles.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Shaukat A, et al. Endoscopic recognition and management strategies for malignant colorectal polyps: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology. 2020;159(5):1916–1934.e2. - PubMed
-
- Merchant J, et al. Concepts and prospects of minimally invasive colorectal cancer surgery. Clin Radiol. 2021;76(12):889–895. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

