Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress
- PMID: 35954150
- PMCID: PMC9367519
- DOI: 10.3390/cells11152306
Hyperoxaluria Induces Endothelial Dysfunction in Preglomerular Arteries: Involvement of Oxidative Stress
Abstract
Urolithiasis is a worldwide problem and a risk factor for kidney injury. Oxidative stress-associated renal endothelial dysfunction secondary to urolithiasis could be a key pathogenic factor, similar to obesity and diabetes-related nephropathy. The aim of the present study was to characterize urolithiasis-related endothelial dysfunction in a hyperoxaluria rat model of renal lithiasis.
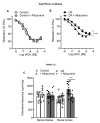
Experimental approach: Endothelial dysfunction was assessed in preglomerular arteries isolated from control rats and in which 0.75% ethylene glycol was administered in drinking water. Renal interlobar arteries were mounted in microvascular myographs for functional studies; superoxide generation was measured by chemiluminescence and mRNA and protein expression by RT-PCR and immunofluorescence, respectively. Selective inhibitors were used to study the influence of the different ROS sources, xanthine oxidase, COX-2, Nox1, Nox2 and Nox4. Inflammatory vascular response was also studied by measuring the RNAm expression of NF-κB, MCP-1 and TNFα by RT-PCR.
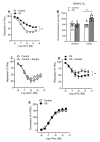
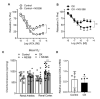
Results: Endothelium-dependent vasodilator responses were impaired in the preglomerular arteries of the hyperoxaluric group along with higher superoxide generation in the renal cortex and vascular inflammation developed by MCP-1 and promoted by NF-κB. The xanthine oxidase inhibitor allopurinol restored the endothelial relaxations and returned superoxide generation to basal values. Nox1 and Nox2 mRNA were up-regulated in arteries from the hyperoxaluric group, and Nox1 and Nox2 selective inhibitors also restored the impaired vasodilator responses and normalized NADPH oxidase-dependent higher superoxide values of renal cortex from the hyperoxaluric group.
Conclusions: The current data support that hyperoxaluria induces oxidative stress-mediated endothelial dysfunction and inflammatory response in renal preglomerular arteries which is promoted by the xanthine oxidase, Nox1 and Nox2 pathways.
Keywords: endothelial dysfunction; oxidative stress; urolithiasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- de Vries A.P., Ruggenenti P., Ruan X.Z., Praga M., Cruzado J.M., Bajema I.M., D’Agati V.D., Lamb H.J., Barlovic D.P., Hojs R., et al. Fatty kidney: Emerging role of ectopic lipid in obesity-related renal disease. Lancet Diabetes Endocrinol. 2014;2:417–426. doi: 10.1016/S2213-8587(14)70065-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

