Immunological Tolerance in Liver Transplant Recipients: Putative Involvement of Neuroendocrine-Immune Interactions
- PMID: 35954171
- PMCID: PMC9367574
- DOI: 10.3390/cells11152327
Immunological Tolerance in Liver Transplant Recipients: Putative Involvement of Neuroendocrine-Immune Interactions
Abstract
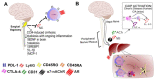
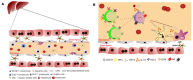
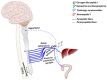
The transplantation world changed significantly following the introduction of immunosuppressants, with millions of people saved. Several physicians have noted that liver recipients that do not take their medication for different reasons became tolerant regarding kidney, heart, and lung transplantations at higher frequencies. Most studies have attempted to explain this phenomenon through unique immunological mechanisms and the fact that the hepatic environment is continuously exposed to high levels of pathogen-associated molecular patterns (PAMPs) or non-pathogenic microorganism-associated molecular patterns (MAMPs) from commensal flora. These components are highly inflammatory in the periphery but tolerated in the liver as part of the normal components that arrive via the hepatic portal vein. These immunological mechanisms are discussed herein based on current evidence, although we hypothesize the participation of neuroendocrine-immune pathways, which have played a relevant role in autoimmune diseases. Cells found in the liver present receptors for several cytokines, hormones, peptides, and neurotransmitters that would allow for system crosstalk. Furthermore, the liver is innervated by the autonomic system and may, thus, be influenced by the parasympathetic and sympathetic systems. This review therefore seeks to discuss classical immunological hepatic tolerance mechanisms and hypothesizes the possible participation of the neuroendocrine-immune system based on the current literature.
Keywords: adrenergic receptor; cholinergic receptor; immunological tolerance; liver transplantation; neuroendocrine-immune interaction; regulatory microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immune-neuroendocrine interactions and autoimmune diseases.Clin Dev Immunol. 2006 Jun-Dec;13(2-4):109-23. doi: 10.1080/17402520600877059. Clin Dev Immunol. 2006. PMID: 17162354 Free PMC article. Review.
-
Sympathoadrenal system in neuroendocrine control of glucose: mechanisms involved in the liver, pancreas, and adrenal gland under hemorrhagic and hypoglycemic stress.Can J Physiol Pharmacol. 1992 Feb;70(2):167-206. doi: 10.1139/y92-024. Can J Physiol Pharmacol. 1992. PMID: 1521177 Review.
-
[Interaction involving the thymus and the hypothalamus-pituitary axis, immunomodulation by hormones].Srp Arh Celok Lek. 2004 May-Jun;132(5-6):187-93. doi: 10.2298/sarh0406187m. Srp Arh Celok Lek. 2004. PMID: 15493593 Review. Serbian.
-
Autonomic nervous system and immune system interactions.Compr Physiol. 2014 Jul;4(3):1177-200. doi: 10.1002/cphy.c130051. Compr Physiol. 2014. PMID: 24944034 Free PMC article. Review.
-
Mechanisms of Immune Tolerance in Liver Transplantation-Crosstalk Between Alloreactive T Cells and Liver Cells With Therapeutic Prospects.Front Immunol. 2019 Nov 19;10:2667. doi: 10.3389/fimmu.2019.02667. eCollection 2019. Front Immunol. 2019. PMID: 31803188 Free PMC article. Review.
Cited by
-
Harnessing Metabolites as Serum Biomarkers for Liver Graft Pathology Prediction Using Machine Learning.Metabolites. 2024 Apr 27;14(5):254. doi: 10.3390/metabo14050254. Metabolites. 2024. PMID: 38786731 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

