Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration
- PMID: 35954178
- PMCID: PMC9367404
- DOI: 10.3390/cells11152334
Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration
Abstract
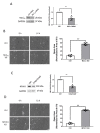
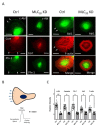
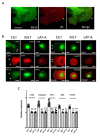
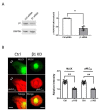
Airway smooth muscle cell migration plays an essential role in airway development, repair, and remodeling. Smooth muscle myosin II has been traditionally thought to localize in the cytoplasm solely and regulates cell migration by affecting stress fiber formation and focal adhesion assembly. In this study, we unexpectedly found that 20-kDa myosin light chain (MLC20) and myosin-11 (MYH11), important components of smooth muscle myosin, were present at the edge of lamellipodia. The knockdown of MLC20 or MYH11 attenuated the recruitment of c-Abl, cortactinProfilin-1 (Pfn-1), and Abi1 to the cell edge. Moreover, myosin light chain kinase (MLCK) colocalized with integrin β1 at the tip of protrusion. The inhibition of MLCK attenuated the recruitment of c-Abl, cortactin, Pfn-1, and Abi1 to the cell edge. Furthermore, MLCK localization at the leading edge was reduced by integrin β1 knockdown. Taken together, our results demonstrate that smooth muscle myosin localizes at the leading edge and orchestrates the recruitment of actin-regulatory proteins to the tip of lamellipodia. Mechanistically, integrin β1 recruits MLCK to the leading edge, which catalyzes MLC20 phosphorylation. Activated myosin regulates the recruitment of actin-regulatory proteins to the leading edge, and promotes lamellipodial formation and migration.
Keywords: actin-associated proteins; leading edge; migration; myosin; smooth muscle.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures


Similar articles
-
Distinctive roles of Abi1 in regulating actin-associated proteins during human smooth muscle cell migration.Sci Rep. 2020 Jun 30;10(1):10667. doi: 10.1038/s41598-020-67781-1. Sci Rep. 2020. PMID: 32606387 Free PMC article.
-
Role of c-Abl tyrosine kinase in smooth muscle cell migration.Am J Physiol Cell Physiol. 2014 Apr 15;306(8):C753-61. doi: 10.1152/ajpcell.00327.2013. Epub 2014 Jan 29. Am J Physiol Cell Physiol. 2014. PMID: 24477238 Free PMC article.
-
MLCK-independent phosphorylation of MLC20 and its regulation by MAP kinase pathway in human bladder smooth muscle cells.Cytoskeleton (Hoboken). 2011 Mar;68(3):139-49. doi: 10.1002/cm.20471. Epub 2010 Aug 18. Cytoskeleton (Hoboken). 2011. PMID: 20722044 Free PMC article.
-
Role of non-kinase activity of myosin light-chain kinase in regulating smooth muscle contraction, a review dedicated to Dr. Setsuro Ebashi.Biochem Biophys Res Commun. 2008 Apr 25;369(1):135-43. doi: 10.1016/j.bbrc.2007.11.096. Epub 2007 Nov 29. Biochem Biophys Res Commun. 2008. PMID: 18053800 Review.
-
Myosin light chain kinase as a multifunctional regulatory protein of smooth muscle contraction.IUBMB Life. 2001 Jun;51(6):337-44. doi: 10.1080/152165401753366087. IUBMB Life. 2001. PMID: 11758800 Review.
Cited by
-
Single-cell RNA sequencing reveals the developmental program underlying proximal-distal patterning of the human lung at the embryonic stage.Cell Res. 2023 Jun;33(6):421-433. doi: 10.1038/s41422-023-00802-6. Epub 2023 Apr 21. Cell Res. 2023. PMID: 37085732 Free PMC article.
References
-
- Freed D.H., Chilton L., Li Y., Dangerfield A.L., Raizman J.E., Rattan S.G., Visen N., Hryshko L.V., Dixon I.M.C. Role of myosin light chain kinase in cardiotrophin-1-induced cardiac myofibroblast cell migration. Am. J. Physiol. Circ. Physiol. 2011;301:H514–H522. doi: 10.1152/ajpheart.01041.2010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

