TGF-β Superfamily Signaling in the Eye: Implications for Ocular Pathologies
- PMID: 35954181
- PMCID: PMC9367584
- DOI: 10.3390/cells11152336
TGF-β Superfamily Signaling in the Eye: Implications for Ocular Pathologies
Abstract
The TGF-β signaling pathway plays a crucial role in several key aspects of development and tissue homeostasis. TGF-β ligands and their mediators have been shown to be important regulators of ocular physiology and their dysregulation has been described in several eye pathologies. TGF-β signaling participates in regulating several key developmental processes in the eye, including angiogenesis and neurogenesis. Inadequate TGF-β signaling has been associated with defective angiogenesis, vascular barrier function, unfavorable inflammatory responses, and tissue fibrosis. In addition, experimental models of corneal neovascularization, diabetic retinopathy, proliferative vitreoretinopathy, glaucoma, or corneal injury suggest that aberrant TGF-β signaling may contribute to the pathological features of these conditions, showing the potential of modulating TGF-β signaling to treat eye diseases. This review highlights the key roles of TGF-β family members in ocular physiology and in eye diseases, and reviews approaches targeting the TGF-β signaling as potential treatment options.
Keywords: BMP; TGF-β; age-related macular degeneration; ocular diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


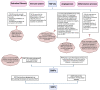
References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Saint-Geniez M., Maharaj A.S., Walshe T.E., Tucker B.A., Sekiyama E., Kurihara T., Darland D.C., Young M.J., D’Amore P.A. Endogenous VEGF is required for visual function: Evidence for a survival role on muller cells and photoreceptors. PLoS ONE. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

