β-Glucocerebrosidase Deficiency Activates an Aberrant Lysosome-Plasma Membrane Axis Responsible for the Onset of Neurodegeneration
- PMID: 35954187
- PMCID: PMC9367513
- DOI: 10.3390/cells11152343
β-Glucocerebrosidase Deficiency Activates an Aberrant Lysosome-Plasma Membrane Axis Responsible for the Onset of Neurodegeneration
Abstract
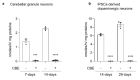
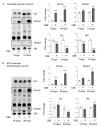
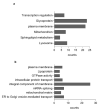
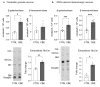
β-glucocerebrosidase is a lysosomal hydrolase involved in the catabolism of the sphingolipid glucosylceramide. Biallelic loss of function mutations in this enzyme are responsible for the onset of Gaucher disease, while monoallelic β-glucocerebrosidase mutations represent the first genetic risk factor for Parkinson's disease. Despite this evidence, the molecular mechanism linking the impairment in β-glucocerebrosidase activity with the onset of neurodegeneration in still unknown. In this frame, we developed two in vitro neuronal models of β-glucocerebrosidase deficiency, represented by mouse cerebellar granule neurons and human-induced pluripotent stem cells-derived dopaminergic neurons treated with the specific β-glucocerebrosidase inhibitor conduritol B epoxide. Neurons deficient for β-glucocerebrosidase activity showed a lysosomal accumulation of glucosylceramide and the onset of neuronal damage. Moreover, we found that neurons react to the lysosomal impairment by the induction of their biogenesis and exocytosis. This latter event was responsible for glucosylceramide accumulation also at the plasma membrane level, with an alteration in lipid and protein composition of specific signaling microdomains. Collectively, our data suggest that β-glucocerebrosidase loss of function impairs the lysosomal compartment, establishing a lysosome-plasma membrane axis responsible for modifications in the plasma membrane architecture and possible alterations of intracellular signaling pathways, leading to neuronal damage.
Keywords: GBA1; Gaucher disease; glucosylceramide; lipid rafts; lysosomes; plasma membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

