Roles of Epigenetics in Cardiac Fibroblast Activation and Fibrosis
- PMID: 35954191
- PMCID: PMC9367448
- DOI: 10.3390/cells11152347
Roles of Epigenetics in Cardiac Fibroblast Activation and Fibrosis
Abstract
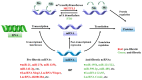
Cardiac fibrosis is a common pathophysiologic process associated with numerous cardiovascular diseases, resulting in cardiac dysfunction. Cardiac fibroblasts (CFs) play an important role in the production of the extracellular matrix and are the essential cell type in a quiescent state in a healthy heart. In response to diverse pathologic stress and environmental stress, resident CFs convert to activated fibroblasts, referred to as myofibroblasts, which produce more extracellular matrix, contributing to cardiac fibrosis. Although multiple molecular mechanisms are implicated in CFs activation and cardiac fibrosis, there is increasing evidence that epigenetic regulation plays a key role in this process. Epigenetics is a rapidly growing field in biology, and provides a modulated link between pathological stimuli and gene expression profiles, ultimately leading to corresponding pathological changes. Epigenetic modifications are mainly composed of three main categories: DNA methylation, histone modifications, and non-coding RNAs. This review focuses on recent advances regarding epigenetic regulation in cardiac fibrosis and highlights the effects of epigenetic modifications on CFs activation. Finally, we provide some perspectives and prospects for the study of epigenetic modifications and cardiac fibrosis.
Keywords: epigenetics; fibroblasts; fibrosis; heart.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

