Piezo1 Channel Activation Reverses Pulmonary Artery Vasoconstriction in an Early Rat Model of Pulmonary Hypertension: The Role of Ca2+ Influx and Akt-eNOS Pathway
- PMID: 35954193
- PMCID: PMC9367624
- DOI: 10.3390/cells11152349
Piezo1 Channel Activation Reverses Pulmonary Artery Vasoconstriction in an Early Rat Model of Pulmonary Hypertension: The Role of Ca2+ Influx and Akt-eNOS Pathway
Abstract
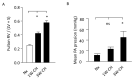
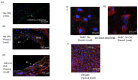
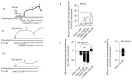
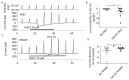
In intrapulmonary arteries (IPAs), mechanical forces due to blood flow control vessel tone, and these forces change during pulmonary hypertension (PH). Piezo1, a stretch-activated calcium channel, is a sensor of mechanical stress present in both endothelial cells (ECs) and smooth muscle cells (SMCs). The present study investigated the role of Piezo1 on IPA in the chronic hypoxia model of PH. Rats were raised in chronically hypoxic conditions for 1 (1W-CH, early stage) or 3 weeks (3W-CH, late-stage) of PH or in normoxic conditions (Nx). Immunofluorescence labeling and patch-clamping revealed the presence of Piezo1 in both ECs and SMCs. The Piezo1 agonist, Yoda1, induced an IPA contraction in Nx and 3W-CH. Conversely, Yoda1 induced an endothelial nitric oxide (eNOS) dependent relaxation in 1W-CH. In ECs, the Yoda1-mediated intracellular calcium concentration ([Ca2+]i) increase was greater in 1W-CH as compared to Nx. Yoda1 induced an EC hyperpolarization in 1W-CH. The eNOS levels were increased in 1W-CH IPA compared to Nx or 3W-CH PH and Yoda1 activated phosphorylation of Akt (Ser473) and eNOS (Ser1177). Thus, we demonstrated that endothelial Piezo1 contributes to intrapulmonary vascular relaxation by controlling endothelial [Ca2+]i, endothelial-dependent hyperpolarization, and Akt-eNOS pathway activation in the early stage of PH.
Keywords: Akt; Piezo channels; calcium signaling; eNOS; pulmonary artery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bonnet S., Dumas-De-La-Roque E., Bégueret H., Marthan R., Fayon M., Dos Santos P., Savineau J.-P., Baulieu E.-E. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc. Natl. Acad. Sci. USA. 2003;100:9488–9493. doi: 10.1073/pnas.1633724100. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

