ENO2 Promotes Colorectal Cancer Metastasis by Interacting with the LncRNA CYTOR and Activating YAP1-Induced EMT
- PMID: 35954207
- PMCID: PMC9367517
- DOI: 10.3390/cells11152363
ENO2 Promotes Colorectal Cancer Metastasis by Interacting with the LncRNA CYTOR and Activating YAP1-Induced EMT
Abstract
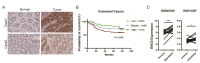
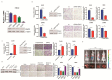
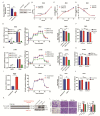
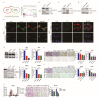
The glycolytic enzyme enolase 2 (ENO2) is dysregulated in many types of cancer. However, the roles and detailed molecular mechanism of ENO2 in colorectal cancer (CRC) metastasis remain unclear. Here, we performed a comprehensive analysis of ENO2 expression in 184 local CRC samples and samples from the TCGA and GEO databases and found that ENO2 upregulation in CRC samples was negatively associated with prognosis. By knocking down and overexpressing ENO2, we found that ENO2 promoted CRC cell migration and invasion, which is dependent on its interaction with the long noncoding RNA (lncRNA) CYTOR, but did not depend on glycolysis regulation. Furthermore, CYTOR mediated ENO2 binding to large tumor suppressor 1 (LATS1) and competitively inhibited the phosphorylation of Yes-associated protein 1 (YAP1), which ultimately triggered epithelial-mesenchymal transition (EMT). Collectively, these findings highlight the molecular mechanism of the ENO2-CYTOR interaction, and ENO2 could be considered a potential therapeutic target for CRC.
Keywords: CYTOR; ENO2; YAP1; colorectal cancer; metastasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Investigation of ENO2 as a promising novel marker for the progression of colorectal cancer with microsatellite instability-high.BMC Cancer. 2024 May 9;24(1):573. doi: 10.1186/s12885-024-12332-4. BMC Cancer. 2024. PMID: 38724951 Free PMC article.
-
The long non-coding RNA CYTOR drives colorectal cancer progression by interacting with NCL and Sam68.Mol Cancer. 2018 Jul 31;17(1):110. doi: 10.1186/s12943-018-0860-7. Mol Cancer. 2018. PMID: 30064438 Free PMC article.
-
SNHG16 upregulation-induced positive feedback loop with YAP1/TEAD1 complex in Colorectal Cancer cell lines facilitates liver metastasis of colorectal cancer by modulating CTCs epithelial-mesenchymal transition.Int J Biol Sci. 2022 Aug 16;18(14):5291-5308. doi: 10.7150/ijbs.73438. eCollection 2022. Int J Biol Sci. 2022. PMID: 36147462 Free PMC article.
-
Long non-coding RNA (lncRNA) and epithelial-mesenchymal transition (EMT) in colorectal cancer: a systematic review.Cancer Biol Ther. 2020 Sep 1;21(9):769-781. doi: 10.1080/15384047.2020.1794239. Epub 2020 Jul 30. Cancer Biol Ther. 2020. PMID: 32730165 Free PMC article.
-
ENO2, a Glycolytic Enzyme, Contributes to Prostate Cancer Metastasis: A Systematic Review of Literature.Cancers (Basel). 2024 Jul 10;16(14):2503. doi: 10.3390/cancers16142503. Cancers (Basel). 2024. PMID: 39061144 Free PMC article. Review.
Cited by
-
ENO2 promotes anoikis resistance in anaplastic thyroid cancer by maintaining redox homeostasis.Gland Surg. 2024 Feb 29;13(2):209-224. doi: 10.21037/gs-24-44. Epub 2024 Feb 23. Gland Surg. 2024. PMID: 38455357 Free PMC article.
-
Investigation of ENO2 as a promising novel marker for the progression of colorectal cancer with microsatellite instability-high.BMC Cancer. 2024 May 9;24(1):573. doi: 10.1186/s12885-024-12332-4. BMC Cancer. 2024. PMID: 38724951 Free PMC article.
-
Systematic identification of pathological mechanisms, prognostic biomarkers and therapeutic targets by integrating lncRNA expression variation in salivary gland mucoepidermoid carcinoma.Sci Rep. 2025 Jan 10;15(1):1573. doi: 10.1038/s41598-025-85535-9. Sci Rep. 2025. PMID: 39794354 Free PMC article.
-
Metabolic enzyme-associated protein-protein interactions (mPPIs) in cancer: potential vulnerability for cancer treatment?Acta Pharmacol Sin. 2025 Jun 20. doi: 10.1038/s41401-025-01601-y. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40542281 Review.
-
High-fat diet driven post-operative colon cancer recurrence is dependent upon genetic susceptibility to deoxycholic acid.Cancer Lett. 2025 Jul 22;631:217943. doi: 10.1016/j.canlet.2025.217943. Online ahead of print. Cancer Lett. 2025. PMID: 40706745
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

