Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors
- PMID: 35954211
- PMCID: PMC9367583
- DOI: 10.3390/cells11152367
Aberrant Expression of COX-2 and FOXG1 in Infrapatellar Fat Pad-Derived ASCs from Pre-Diabetic Donors
Abstract
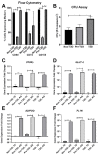
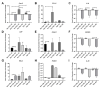
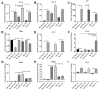
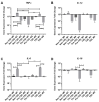
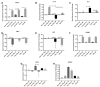
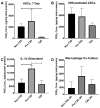
Osteoarthritis (OA) is a degenerative joint disease resulting in limited mobility and severe disability. Type II diabetes mellitus (T2D) is a weight-independent risk factor for OA, but a link between the two diseases has not been elucidated. Adipose stem cells (ASCs) isolated from the infrapatellar fat pad (IPFP) may be a viable regenerative cell for OA treatment. This study analyzed the expression profiles of inflammatory and adipokine-related genes in IPFP-ASCs of non-diabetic (Non-T2D), pre-diabetic (Pre-T2D), and T2D donors. Pre-T2D ASCs exhibited a substantial decrease in levels of mesenchymal markers CD90 and CD105 with no change in adipogenic differentiation compared to Non-T2D and T2D IPFP-ASCs. In addition, Cyclooxygenase-2 (COX-2), Forkhead box G1 (FOXG1) expression and prostaglandin E2 (PGE2) secretion were significantly increased in Pre-T2D IPFP-ASCs upon stimulation by interleukin-1 beta (IL-1β). Interestingly, M1 macrophages exhibited a significant reduction in expression of pro-inflammatory markers TNFα and IL-6 when co-cultured with Pre-T2D IPFP-ASCs. These data suggest that the heightened systemic inflammation associated with untreated T2D may prime the IPFP-ASCs to exhibit enhanced anti-inflammatory characteristics via suppressing the IL-6/COX-2 signaling pathway. In addition, the elevated production of PGE2 by the Pre-T2D IPFP-ASCs may also suggest the contribution of pre-diabetic conditions to the onset and progression of OA.
Keywords: COX-2; FOXG1; adipose stem cell; diabetes; infrapatellar fat pad; osteoarthritis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Deshpande B.R., Katz J.N., Solomon D.H., Yelin E.H., Hunter D.J., Messier S.P., Suter L.G., Losina E. Number of persons with symptomatic knee osteoarthritis in the US: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016;68:1743–1750. doi: 10.1002/acr.22897. - DOI - PMC - PubMed
-
- Cross M., Smith E., Hoy D., Nolte S., Ackerman I., Fransen M., Bridgett L., Williams S., Guillemin F., Hill C.L., et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. - DOI - PubMed
-
- Goldring S.R., Goldring M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J. Musculoskelet. Neuronal. Interact. 2006;6:376–378. - PubMed
-
- Bondeson J., Wainwright S.D., Lauder S., Amos N., Hughes C.E. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res. Ther. 2006;8:R187. doi: 10.1186/ar2099. - DOI - PMC - PubMed
-
- Song I.H., Althoff C.E., Hermann K.G., Scheel A.K., Knetsch T., Schoenharting M., Werner C., Burmester G.R., Backhaus M. Knee osteoarthritis. Efficacy of a new method of contrast-enhanced musculoskeletal ultrasonography in detection of synovitis in patients with knee osteoarthritis in comparison with magnetic resonance imaging. Ann. Rheum. Dis. 2008;67:19–25. doi: 10.1136/ard.2006.067462. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

