Rewiring of Glucose and Lipid Metabolism Induced by G Protein-Coupled Receptor 17 Silencing Enables the Transition of Oligodendrocyte Progenitors to Myelinating Cells
- PMID: 35954217
- PMCID: PMC9368002
- DOI: 10.3390/cells11152369
Rewiring of Glucose and Lipid Metabolism Induced by G Protein-Coupled Receptor 17 Silencing Enables the Transition of Oligodendrocyte Progenitors to Myelinating Cells
Abstract
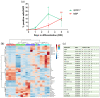
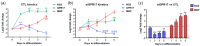
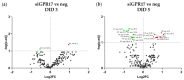
In the mature central nervous system (CNS), oligodendrocytes (OLs) provide support and insulation to axons thanks to the production of a myelin sheath. During their maturation to myelinating cells, OLs require energy and building blocks for lipids, which implies a great investment of energy fuels and molecular sources of carbon. The oligodendroglial G protein-coupled receptor 17 (GPR17) has emerged as a key player in OL maturation; it reaches maximal expression in pre-OLs, but then it has to be internalized to allow terminal maturation. In this study, we aim at elucidating the role of physiological GPR17 downregulation in OL metabolism by applying transcriptomics, metabolomics and lipidomics on differentiating OLs. After GPR17 silencing, we found a significant increase in mature OL markers and alteration of several genes involved in glucose metabolism and lipid biosynthesis. We also observed an increased release of lactate, which is partially responsible for the maturation boost induced by GPR17 downregulation. Concomitantly, GPR17 depletion also changed the kinetics of specific myelin lipid classes. Globally, this study unveils a functional link between GPR17 expression, lactate release and myelin composition, and suggests that innovative interventions targeting GPR17 may help to foster endogenous myelination in demyelinating diseases.
Keywords: energy metabolism; glycolysis; lactate; lipidomics; metabolomics; myelin lipids; myelination; oligodendrocyte; oligodendrocyte progenitor cell; transcriptomics.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Rao V.T.S., Khan D., Cui Q.L., Fuh S.C., Hossain S., Almazan G., Multhaup G., Healy L.M., Kennedy T.E., Antel J.P. Distinct age and differentiation-state dependent metabolic profiles of oligodendrocytes under optimal and stress conditions. PLoS ONE. 2017;12:e0182372. doi: 10.1371/journal.pone.0182372. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

