The AGEs/RAGE Transduction Signaling Prompts IL-8/CXCR1/2-Mediated Interaction between Cancer-Associated Fibroblasts (CAFs) and Breast Cancer Cells
- PMID: 35954247
- PMCID: PMC9368521
- DOI: 10.3390/cells11152402
The AGEs/RAGE Transduction Signaling Prompts IL-8/CXCR1/2-Mediated Interaction between Cancer-Associated Fibroblasts (CAFs) and Breast Cancer Cells
Abstract
Advanced glycation end products (AGEs) and the cognate receptor, named RAGE, are involved in metabolic disorders characterized by hyperglycemia, type 2 diabetes mellitus (T2DM) and obesity. Moreover, the AGEs/RAGE transduction pathway prompts a dysfunctional interaction between breast cancer cells and tumor stroma toward the acquisition of malignant features. However, the action of the AGEs/RAGE axis in the main players of the tumor microenvironment, named breast cancer-associated fibroblasts (CAFs), remains to be fully explored. In the present study, by chemokine array, we first assessed that interleukin-8 (IL-8) is the most up-regulated pro-inflammatory chemokine upon AGEs/RAGE activation in primary CAFs, obtained from breast tumors. Thereafter, we ascertained that the AGEs/RAGE signaling promotes a network cascade in CAFs, leading to the c-Fos-dependent regulation of IL-8. Next, using a conditioned medium from AGEs-exposed CAFs, we determined that IL-8/CXCR1/2 paracrine activation induces the acquisition of migratory and invasive features in MDA-MB-231 breast cancer cells. Altogether, our data provide new insights on the involvement of IL-8 in the AGEs/RAGE transduction pathway among the intricate connections linking breast cancer cells to the surrounding stroma. Hence, our findings may pave the way for further investigations to define the role of IL-8 as useful target for the better management of breast cancer patients exhibiting metabolic disorders.
Keywords: AGEs; IL-8; RAGE; breast cancer; cancer-associated fibroblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
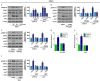


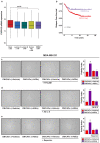
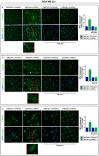
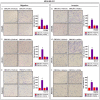
References
-
- Vella V., De Francesco E.M., Lappano R., Muoio M.G., Manzella L., Maggiolini M., Belfiore A. Microenvironmental Determinants of Breast Cancer Metastasis: Focus on the Crucial Interplay Between Estrogen and Insulin/Insulin-Like Growth Factor Signaling. Front. Cell Dev. Biol. 2020;8:608412. doi: 10.3389/fcell.2020.608412. - DOI - PMC - PubMed
-
- Buono G., Crispo A., Giuliano M., de Angelis C., Schettini F., Forestieri V., Lauria R., Pensabene M., de Laurentiis M., Augustin L.S.A., et al. Combined effect of obesity and diabetes on early breast cancer outcome: A prospective observational study. Oncotarget. 2017;8:115709–115717. doi: 10.18632/oncotarget.22977. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

