Retinal Vascular Physiology Biomarkers in a 5XFAD Mouse Model of Alzheimer's Disease
- PMID: 35954257
- PMCID: PMC9368483
- DOI: 10.3390/cells11152413
Retinal Vascular Physiology Biomarkers in a 5XFAD Mouse Model of Alzheimer's Disease
Abstract
Background: Alzheimer's disease (AD) is a neurodegenerative disorder that affects the brain and retina and lacks reliable biomarkers for early diagnosis. As amyloid beta (Aβ) manifestations emerge prior to clinical symptoms and plaques of amyloid may cause vascular damage, identification of retinal vascular biomarkers may improve knowledge of AD pathophysiology and potentially serve as therapeutic targets. The purpose of the current study was to test the hypothesis that retinal hemodynamic and oxygen metrics are altered in 5XFAD mice.
Methods: Thirty-two male mice were evaluated at 3 months of age: sixteen 5XFAD transgenic and sixteen wild-type mice. Spectral-domain optical coherence tomography, vascular oxygen tension, and blood flow imaging were performed in one eye of each mouse. After imaging, the imaged and fellow retinal tissues were submitted for histological sectioning and amyloid protein analysis, respectively. Protein analysis was also performed on the brain tissues.
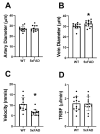
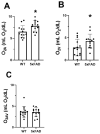
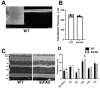
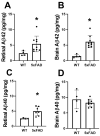
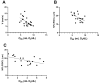
Results: Retinal physiological changes in venous diameter and blood velocity, arterial and venous oxygen contents, coupled with anatomical alterations in the thickness of retinal cell layers were detected in 5XFAD mice. Moreover, an increase in Aβ42 levels in both the retina and brain tissues was observed in 5XFAD mice. Significant changes in retinal oxygen delivery, metabolism, or extraction fraction were not detected. Based on compiled data from both groups, arterial oxygen content was inversely related to venous blood velocity and nerve fiber/ganglion cell layer thickness.
Conclusions: Concurrent alterations in retinal hemodynamic and oxygen metrics, thickness, and tissue Aβ42 protein levels in 5XFAD mice at 3 months of age corresponded to previously reported findings in human AD. Overall, these results suggest that this mouse model can be utilized for studying pathophysiology of AD and evaluating potential therapies.
Keywords: 5XFAD; Alzheimer’s disease; amyloid β; retinal vascular physiology biomarkers.
Conflict of interest statement
MS holds a patent for the oxygen imaging technology. The other authors have no conflict of interest.
Figures

References
-
- Martin J.P. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International; London, UK: 2015.
-
- Oakley H., Cole S.L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

