Taste Receptor Activation in Tracheal Brush Cells by Denatonium Modulates ENaC Channels via Ca2+, cAMP and ACh
- PMID: 35954259
- PMCID: PMC9367940
- DOI: 10.3390/cells11152411
Taste Receptor Activation in Tracheal Brush Cells by Denatonium Modulates ENaC Channels via Ca2+, cAMP and ACh
Abstract
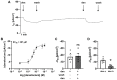
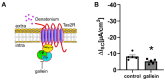
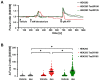
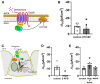
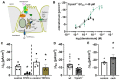
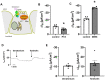
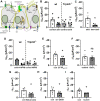
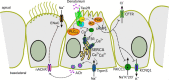
Mucociliary clearance is a primary defence mechanism of the airways consisting of two components, ciliary beating and transepithelial ion transport (ISC). Specialised chemosensory cholinergic epithelial cells, named brush cells (BC), are involved in regulating various physiological and immunological processes. However, it remains unclear if BC influence ISC. In murine tracheae, denatonium, a taste receptor agonist, reduced basal ISC in a concentration-dependent manner (EC50 397 µM). The inhibition of bitter taste signalling components with gallein (Gβγ subunits), U73122 (phospholipase C), 2-APB (IP3-receptors) or with TPPO (Trpm5, transient receptor potential-melastatin 5 channel) reduced the denatonium effect. Supportively, the ISC was also diminished in Trpm5-/- mice. Mecamylamine (nicotinic acetylcholine receptor, nAChR, inhibitor) and amiloride (epithelial sodium channel, ENaC, antagonist) decreased the denatonium effect. Additionally, the inhibition of Gα subunits (pertussis toxin) reduced the denatonium effect, while an inhibition of phosphodiesterase (IBMX) increased and of adenylate cyclase (forskolin) reversed the denatonium effect. The cystic fibrosis transmembrane conductance regulator (CFTR) inhibitor CFTRinh172 and the KCNQ1 potassium channel antagonist chromanol 293B both reduced the denatonium effect. Thus, denatonium reduces ISC via the canonical bitter taste signalling cascade leading to the Trpm5-dependent nAChR-mediated inhibition of ENaC as well as Gα signalling leading to a reduction in cAMP-dependent ISC. Therefore, BC activation contributes to the regulation of fluid homeostasis.
Keywords: ACh; CFTR; ENaC; Trpm5; Ussing chamber; airway epithelium; brush cell; ion transport; mucociliary clearance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Bitter taste receptor agonists regulate epithelial two-pore potassium channels via cAMP signaling.Respir Res. 2021 Jan 28;22(1):31. doi: 10.1186/s12931-021-01631-0. Respir Res. 2021. PMID: 33509163 Free PMC article.
-
Bitter taste transduction of denatonium in the mudpuppy Necturus maculosus.J Neurosci. 1997 May 15;17(10):3580-7. doi: 10.1523/JNEUROSCI.17-10-03580.1997. J Neurosci. 1997. PMID: 9133381 Free PMC article.
-
Tracheal brush cells release acetylcholine in response to bitter tastants for paracrine and autocrine signaling.FASEB J. 2020 Jan;34(1):316-332. doi: 10.1096/fj.201901314RR. Epub 2019 Nov 22. FASEB J. 2020. PMID: 31914675
-
ENaC inhibitors and airway re-hydration in cystic fibrosis: state of the art.Curr Mol Pharmacol. 2013 Mar;6(1):3-12. doi: 10.2174/18744672112059990025. Curr Mol Pharmacol. 2013. PMID: 23547930 Review.
-
Pharmacotherapy of the ion transport defect in cystic fibrosis: role of purinergic receptor agonists and other potential therapeutics.Am J Respir Med. 2003;2(4):299-309. doi: 10.1007/BF03256658. Am J Respir Med. 2003. PMID: 14719996 Review.
Cited by
-
Tuft cells in the intestine, immunity and beyond.Nat Rev Gastroenterol Hepatol. 2024 Dec;21(12):852-868. doi: 10.1038/s41575-024-00978-1. Epub 2024 Sep 26. Nat Rev Gastroenterol Hepatol. 2024. PMID: 39327439 Review.
-
Generation and functional characterization of tuft cells in non-human primate pancreatic ducts through organoid culture systems.Front Cell Dev Biol. 2025 May 6;13:1593226. doi: 10.3389/fcell.2025.1593226. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40395933 Free PMC article.
-
Tuft cell-derived acetylcholine promotes epithelial chloride secretion and intestinal helminth clearance.Immunity. 2024 Jun 11;57(6):1243-1259.e8. doi: 10.1016/j.immuni.2024.03.023. Epub 2024 May 13. Immunity. 2024. PMID: 38744291 Free PMC article.
-
Tracheal tuft cells release ATP and link innate to adaptive immunity in pneumonia.Nat Commun. 2025 Jan 10;16(1):584. doi: 10.1038/s41467-025-55936-5. Nat Commun. 2025. PMID: 39794305 Free PMC article.
-
Tuft cell-derived acetylcholine regulates epithelial fluid secretion.bioRxiv [Preprint]. 2023 Mar 22:2023.03.17.533208. doi: 10.1101/2023.03.17.533208. bioRxiv. 2023. Update in: Immunity. 2024 Jun 11;57(6):1243-1259.e8. doi: 10.1016/j.immuni.2024.03.023. PMID: 36993541 Free PMC article. Updated. Preprint.
References
-
- Song Y., Namkung W., Nielson D.W., Lee J.W., Finkbeiner W.E., Verkman A.S. Airway surface liquid depth measured in ex vivo fragments of pig and human trachea: Dependence on Na+ and Cl− channel function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009;297:1131–1140. doi: 10.1152/ajplung.00085.2009. - DOI - PMC - PubMed
-
- Flagella M., Clarke L.L., Miller M.L., Erway L.C., Giannella R.A., Andringa A., Gawenis L.R., Kramer J., Duffy J.J., Doetschman T., et al. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J. Biol. Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

