High Levels of Tumor miR-187-3p-A Potential Tumor-Suppressor microRNA-Are Correlated with Poor Prognosis in Colorectal Cancer
- PMID: 35954265
- PMCID: PMC9367907
- DOI: 10.3390/cells11152421
High Levels of Tumor miR-187-3p-A Potential Tumor-Suppressor microRNA-Are Correlated with Poor Prognosis in Colorectal Cancer
Abstract
Background: The microRNA miR-187-3p plays antitumor roles in a variety of cancers. We and others have previously identified miR-187-3p as a potential tumor suppressor in colorectal cancer (CRC), but there are also reports revealing that high miR-187-3p levels are associated with poor prognosis among CRC patients. This study further investigated the clinicopathological significance of miR-187-3p in CRC.
Methods: MiR-187-3p levels in paired polyp/CRC/normal specimens or primary CRC/liver metastasis specimens were determined by qPCR, and correlated with the patient's clinicopathological and postoperative survival data. The clinical findings were validated using our validation cohort and data obtained from the TCGA or GEO databases. The functional effects of miR-187-3p were investigated through its overexpression in CRC cell lines.
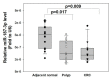
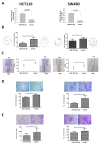
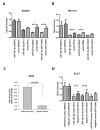
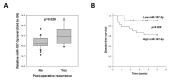
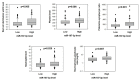
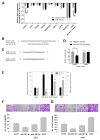
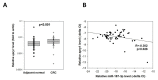
Results: MiR-187-3p was significantly repressed in colorectal polyps and CRC when compared to adjacent normal tissue. Overexpression of miR-187-3p in CRC cell lines impaired colony formation, cell migration, and invasion, and induced chemosensitivity. Clinical analysis revealed that despite miR-187-3p being repressed in CRC, high tumor miR-187-3p levels were positively correlated with tumor stage and disease recurrence. Further analysis showed that miR-187-3p levels were lower in metastatic specimens when compared to paired primary CRC, suggesting that high tumor miR-187-3p levels resulted from the dissemination of metastatic tumor cells. Tumor miR-187-3p levels were positively correlated with peripheral inflammation-related blood markers. Finally, SPRY1 was identified as a novel target gene of miR-187-3p, and was involved in miR-187-3p-impaired CRC metastasis.
Conclusions: This study demonstrated that in spite of its repression and role as a tumor suppressor in CRC, high levels of miR-187-3p in tumors were correlated with poor prognosis and higher levels of peripheral inflammation-related blood markers.
Keywords: colorectal cancer; miR-187-3p; neutrophil; platelet; tumor suppressor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

