Analysis of Genomic Alterations Associated with Recurrence in Early Stage HER2-Positive Breast Cancer
- PMID: 35954313
- PMCID: PMC9367395
- DOI: 10.3390/cancers14153650
Analysis of Genomic Alterations Associated with Recurrence in Early Stage HER2-Positive Breast Cancer
Abstract
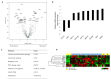
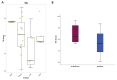
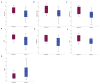
We aimed to compare gene expression in primary tumors of patients with recurrence and nonrecurrence to gain insight into the biology of high-risk HER2-positive early breast cancer. Patients who underwent curative resection and received adjuvant trastuzumab for HER2-positive early breast cancer were evaluated. Gene expression analyses were performed using NanoString Technologies’ nCounter Breast Cancer 360 Panel. PAM50 intrinsic subtypes and Breast Cancer Signatures including tumor inflammation signature (TIS) were evaluated. Of 247 patients, 28 (11.3%) had recurrence at a median follow-up of 54.2 months. Patients with pathological stage III, tumor size > 5 cm, axillary lymph node metastases, and hormone receptor-negativity were more frequently observed in the recurrent group compared with the nonrecurrent group. In patients with recurrence, seven genes were upregulated significantly, including WNT11, HAPLN1, FGF10, BBOX1, CXADR, NDP, and EREG, and two genes were downregulated, including CXCL9 and GNLY. TIS score was significantly lower in patients with recurrence compared with controls without recurrence. These findings suggest that activation of oncogenic signaling pathways related to cell proliferation, adhesion, cancer stemness, and noninflamed tumor microenvironment are associated with the risk of recurrence in early stage, HER2-positive breast cancer.
Keywords: breast cancer; human epidermal growth factor receptor 2; recurrence; tumor inflammation signature.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

