Leydig Cell Tumors of the Testis: An Update of the Imaging Characteristics of a Not So Rare Lesion
- PMID: 35954321
- PMCID: PMC9367522
- DOI: 10.3390/cancers14153652
Leydig Cell Tumors of the Testis: An Update of the Imaging Characteristics of a Not So Rare Lesion
Abstract
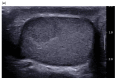
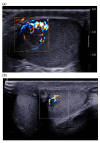
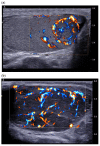
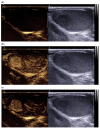
Pre-operative testicular tumor characterization is a challenge for radiologists and urologists. New data concerning imaging approaches or immunochemistry markers improve the management of patients presenting with a testicular tumor, sometimes avoiding radical orchiectomy. In the past 20 years, imaging modalities, especially ultrasound (US) and magnetic resonance imaging (MRI), improved, allowing for great progress in lesion characterization. Leydig cell tumors (LCT) are rare testicular tumors developing from the stromal tissue, with relatively scarce literature, as most of the studies focus on the much more frequent germ cell tumors. However, with the increase in testicular sonography numbers, the incidence of LCT appears much higher than expected, with some studies reporting up to 22% of small testicular nodules. Multimodal ultrasound using Doppler, Elastography, or injection of contrast media can provide crucial arguments to differentiate LCT from germ cell tumors. Multiparametric MRI is a second intention exam, but it allows for extraction of quantifiable data to assess the diagnosis of LCT. The aims of this article are to review the latest data regarding LCT imaging features, using multimodal ultrasound and multiparametric MRI, and to focus on the peculiar aspect of the testis of patients with Klinefelter's syndrome. The possibility of an LCT should be raised in front of a small hypoechoic tumor with a marked corbelling hypervascularization in an otherwise normal testicular pulp. Ultrasonographic modules, such as ultrasensitive Doppler, contrast-enhanced ultrasonography, or elastography, can be used to reinforce the suspicion of LCT. MRI provides objective data regarding vascularization and enhancement kinetics.
Keywords: Doppler; Klinefelter’s syndrome (KS); Leydig cell hyperplasia; Leydig cell tumors (LCT); contrast-enhanced ultrasound (CEUS); elastography; magnetic resonance imaging (MRI); testicular stromal tumors; ultrasound (US).
Conflict of interest statement
The Authors declare no conflict of interest.
Figures

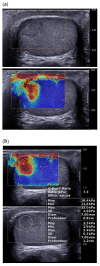
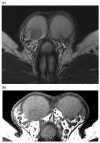

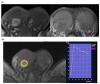
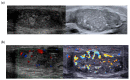
References
-
- Isidori A.M., Pozza C., Gianfrilli D., Giannetta E., Lemma A., Pofi R., Barbagallo F., Manganaro L., Martino G., Lombardo F., et al. Differential Diagnosis of Nonpalpable Testicular Lesions: Qualitative and Quantitative Contrast-enhanced US of Benign and Malignant Testicular Tumors. Radiology. 2014;273:606–618. doi: 10.1148/radiol.14132718. - DOI - PubMed
-
- Colecchia M., Nistal M., Gonzalez-Peramato P., Carmignani L., Salvioni R., Nicolai N., Regadera J. Leydig cell tumor and hyperplasia: A review. Anal. Quant. Cytol. Histol. 2007;29:139–147. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

