Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study
- PMID: 35954356
- PMCID: PMC9367430
- DOI: 10.3390/cancers14153691
Rearrangements, Expression, and Clinical Significance of MYB and MYBL1 in Adenoid Cystic Carcinoma: A Multi-Institutional Study
Abstract
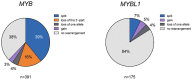
Adenoid cystic carcinoma (ACC) is an aggressive head and neck malignancy characterized by a t (6;9) translocation resulting in an MYB-NFIB gene fusion or, more rarely, an MYBL1 fusion. The true frequency and clinical significance of these alterations are still unclear. Here, we have used tissue microarrays and analyzed 391 ACCs and 647 non-ACC salivary neoplasms to study the prevalence, expression, and clinical significance of MYB/MYBL1 alterations by FISH and immunohistochemistry. Alterations of MYB or MYBL1 were found in 78% of the cases, of which 62% had MYB alterations and 16% had MYBL1 rearrangements. Overexpression of MYB/MYBL1 oncoproteins was detected in 93% of the cases. MYB split signal, seen in 39% of the cases, was specific for ACC and not encountered in non-ACC salivary tumors. Loss of the 3'-part of MYB was enriched in grade 3 tumors and was a significant independent prognostic biomarker for overall survival in multivariate analyses. We hypothesize that loss of the 3'-part of MYB results from an unbalanced t(6;9) leading to an MYB-NFIB fusion with concomitant loss of the segment distal to the MYB breakpoint in 6q23.3. Our study provides new knowledge about the prevalence and clinical significance of MYB/MYBL1 alterations and indicates the presence of genes with tumor suppressive functions in 6q23.3-qter that contribute to poor prognosis and short overall survival in ACC.
Keywords: FISH; MYB; MYBL1; adenoid cystic carcinoma; immunohistochemistry; prognostic biomarker; tumor suppressor gene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Novel MYBL1 Gene Rearrangements with Recurrent MYBL1-NFIB Fusions in Salivary Adenoid Cystic Carcinomas Lacking t(6;9) Translocations.Clin Cancer Res. 2016 Feb 1;22(3):725-33. doi: 10.1158/1078-0432.CCR-15-2867-T. Epub 2015 Dec 2. Clin Cancer Res. 2016. PMID: 26631609 Free PMC article.
-
MYB and MYBL1 in adenoid cystic carcinoma: diversity in the mode of genomic rearrangement and transcripts.Mod Pathol. 2018 Jun;31(6):934-946. doi: 10.1038/s41379-018-0008-8. Epub 2018 Feb 6. Mod Pathol. 2018. PMID: 29410490
-
Possible Relationship Between MYBL1 Alterations and Specific Primary Sites in Adenoid Cystic Carcinoma: A Clinicopathological and Molecular Study of 36 Cases.Yonago Acta Med. 2019 Mar 28;62(1):67-76. doi: 10.33160/yam.2019.03.010. eCollection 2019 Mar. Yonago Acta Med. 2019. PMID: 30962747 Free PMC article.
-
Adenoid cystic carcinoma: emerging role of translocations and gene fusions.Oncotarget. 2016 Oct 4;7(40):66239-66254. doi: 10.18632/oncotarget.11288. Oncotarget. 2016. PMID: 27533466 Free PMC article. Review.
-
Molecular pathology of adenoid cystic carcinoma.Histol Histopathol. 2025 Apr 2:18915. doi: 10.14670/HH-18-915. Online ahead of print. Histol Histopathol. 2025. PMID: 40254959 Review.
Cited by
-
The mitotic checkpoint kinase BUB1 is a direct and actionable target of MYB in adenoid cystic carcinoma.FEBS Lett. 2024 Jan;598(2):252-265. doi: 10.1002/1873-3468.14786. Epub 2023 Dec 27. FEBS Lett. 2024. PMID: 38112379 Free PMC article.
-
Reversible downregulation of HLA class I in adenoid cystic carcinoma.J Immunother Cancer. 2025 Apr 20;13(4):e011380. doi: 10.1136/jitc-2024-011380. J Immunother Cancer. 2025. PMID: 40254393 Free PMC article.
-
Molecular Aspects of Mucoepidermoid Carcinoma and Adenoid Cystic Carcinoma of the Salivary Gland.Head Neck Pathol. 2024 Apr 24;18(1):34. doi: 10.1007/s12105-024-01629-2. Head Neck Pathol. 2024. PMID: 38658430 Free PMC article. Review.
-
Salivary glands adenoid cystic carcinoma: a molecular profile update and potential implications.Front Oncol. 2023 Jul 5;13:1191218. doi: 10.3389/fonc.2023.1191218. eCollection 2023. Front Oncol. 2023. PMID: 37476370 Free PMC article. Review.
-
The evolving landscape of salivary gland tumors.CA Cancer J Clin. 2023 Nov-Dec;73(6):597-619. doi: 10.3322/caac.21807. Epub 2023 Jul 25. CA Cancer J Clin. 2023. PMID: 37490348 Free PMC article. Review.
References
-
- Stenman G., Licitra L., Said-Al-Naief N., van Zante A., Yarbrough W.G. Adenoid cystic carcinoma. In: El-Naggar A.K., Chan J.K.C., Grandis J.R., Takata T., Slootweg P.J., editors. World Health Organization Classification of Head and Neck Tumours. IARC Press; Lyon, France: 2017. pp. 164–165.
-
- Xu B., Drill E., Ho A., Ho A., Dunn L., Prieto-Granada C.N., Chan T., Ganly I., Ghossein R., Katabi N. Predictors of Outcome in Adenoid Cystic Carcinoma of Salivary Glands: A Clinicopathologic Study with Correlation Between MYB Fusion and Protein Expression. Am. J. Surg. Pathol. 2017;41:1422–1432. doi: 10.1097/PAS.0000000000000918. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

