Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy
- PMID: 35954407
- PMCID: PMC9367289
- DOI: 10.3390/cancers14153743
Molecular Pathways and Genomic Landscape of Glioblastoma Stem Cells: Opportunities for Targeted Therapy
Abstract
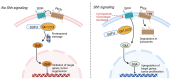
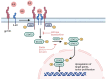
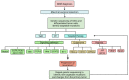
Glioblastoma (GBM) is an aggressive tumor of the central nervous system categorized by the World Health Organization as a Grade 4 astrocytoma. Despite treatment with surgical resection, adjuvant chemotherapy, and radiation therapy, outcomes remain poor, with a median survival of only 14-16 months. Although tumor regression is often observed initially after treatment, long-term recurrence or progression invariably occurs. Tumor growth, invasion, and recurrence is mediated by a unique population of glioblastoma stem cells (GSCs). Their high mutation rate and dysregulated transcriptional landscape augment their resistance to conventional chemotherapy and radiation therapy, explaining the poor outcomes observed in patients. Consequently, GSCs have emerged as targets of interest in new treatment paradigms. Here, we review the unique properties of GSCs, including their interactions with the hypoxic microenvironment that drives their proliferation. We discuss vital signaling pathways in GSCs that mediate stemness, self-renewal, proliferation, and invasion, including the Notch, epidermal growth factor receptor, phosphatidylinositol 3-kinase/Akt, sonic hedgehog, transforming growth factor beta, Wnt, signal transducer and activator of transcription 3, and inhibitors of differentiation pathways. We also review epigenomic changes in GSCs that influence their transcriptional state, including DNA methylation, histone methylation and acetylation, and miRNA expression. The constituent molecular components of the signaling pathways and epigenomic regulators represent potential sites for targeted therapy, and representative examples of inhibitory molecules and pharmaceuticals are discussed. Continued investigation into the molecular pathways of GSCs and candidate therapeutics is needed to discover new effective treatments for GBM and improve survival.
Keywords: glioblastoma; molecular pathway; stem cell; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Understanding the Genomic Landscape of Glioblastoma: Opportunities for Targeted Therapies.Anticancer Res. 2024 Nov;44(11):4677-4690. doi: 10.21873/anticanres.17295. Anticancer Res. 2024. PMID: 39477295 Review.
-
Dihydropyrimidinase-related protein 5 controls glioblastoma stem cell characteristics as a biomarker of proneural-subtype glioblastoma stem cells.Oncol Lett. 2020 Aug;20(2):1153-1162. doi: 10.3892/ol.2020.11668. Epub 2020 May 22. Oncol Lett. 2020. PMID: 32724355 Free PMC article.
-
Targeting Glioblastoma Stem Cells: A Review on Biomarkers, Signal Pathways and Targeted Therapy.Front Oncol. 2021 Jul 8;11:701291. doi: 10.3389/fonc.2021.701291. eCollection 2021. Front Oncol. 2021. PMID: 34307170 Free PMC article. Review.
-
Growth factor independence underpins a paroxysmal, aggressive Wnt5aHigh/EphA2Low phenotype in glioblastoma stem cells, conducive to experimental combinatorial therapy.J Exp Clin Cancer Res. 2022 Apr 12;41(1):139. doi: 10.1186/s13046-022-02333-1. J Exp Clin Cancer Res. 2022. PMID: 35414102 Free PMC article.
-
MicroRNA-137 is downregulated in glioblastoma and inhibits the stemness of glioma stem cells by targeting RTVP-1.Oncotarget. 2013 May;4(5):665-76. doi: 10.18632/oncotarget.928. Oncotarget. 2013. PMID: 23714687 Free PMC article.
Cited by
-
Dendrimer Technology in Glioma: Functional Design and Potential Applications.Cancers (Basel). 2023 Feb 8;15(4):1075. doi: 10.3390/cancers15041075. Cancers (Basel). 2023. PMID: 36831418 Free PMC article. Review.
-
Synergistic Dual Targeting of Thioredoxin and Glutathione Systems Irrespective of p53 in Glioblastoma Stem Cells.Antioxidants (Basel). 2024 Oct 3;13(10):1201. doi: 10.3390/antiox13101201. Antioxidants (Basel). 2024. PMID: 39456455 Free PMC article.
-
Emerging Approaches in Glioblastoma Treatment: Modulating the Extracellular Matrix Through Nanotechnology.Pharmaceutics. 2025 Jan 21;17(2):142. doi: 10.3390/pharmaceutics17020142. Pharmaceutics. 2025. PMID: 40006509 Free PMC article. Review.
-
Nrf2 signaling pathway: current status and potential therapeutic targetable role in human cancers.Front Oncol. 2023 Sep 22;13:1184079. doi: 10.3389/fonc.2023.1184079. eCollection 2023. Front Oncol. 2023. PMID: 37810967 Free PMC article. Review.
-
Osmotic Pressure and Its Biological Implications.Int J Mol Sci. 2024 Mar 14;25(6):3310. doi: 10.3390/ijms25063310. Int J Mol Sci. 2024. PMID: 38542282 Free PMC article. Review.
References
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

