Clinical Post-SARS-CoV-2 Infection Scenarios in Vaccinated and Non-Vaccinated Cancer Patients in Three German Cancer Centers: A Retrospective Analysis
- PMID: 35954410
- PMCID: PMC9367483
- DOI: 10.3390/cancers14153746
Clinical Post-SARS-CoV-2 Infection Scenarios in Vaccinated and Non-Vaccinated Cancer Patients in Three German Cancer Centers: A Retrospective Analysis
Abstract
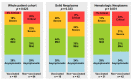
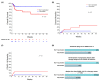
COVID-19 vaccines have become an integral element in the protection of cancer patients against SARS-CoV-2. To date, there are no direct comparisons of the course of COVID-19 infection in cancer patients between the pre- and post-vaccine era. We analyzed SARS-CoV-2 infections and their impact on cancer in COVID-19 vaccinated and non-vaccinated patients from three German cancer centers. Overall, 133 patients with SARS-CoV-2 were enrolled in pre- and post-vaccine eras: 84 non-vaccinated and 49 vaccinated, respectively. A mild course of COVID-19 was documented more frequently in vaccinated patients (49% vs. 29%), while the frequency of severe and critical courses occurred in approximately one-half of the non-vaccinated patients (22% vs. 42%, p = 0.023). Particularly, patients with hematologic neoplasms benefited from vaccination in this context (p = 0.031). Admissions to intermediate- and intensive-care units and the necessity of non-invasive and invasive respiratory support were reduced by 71% and 50% among vaccinated patients, respectively. The median length of admission was 11 days for non-vaccinated and 5 days for vaccinated patients (p = 0.002). COVID-19 mortality was reduced by 83% in vaccinated patients (p = 0.046). Finally, the median time from SARS-CoV-2 infection to restarting cancer therapy was 12 and 26 days among vaccinated and non-vaccinated groups, respectively (p = 0.002). Although this study does not have enough power to perform multivariate analyses to account for confounders, it provides data on COVID-19 in non-vaccinated and vaccinated cancer patients and illustrates the potential benefits of COVID-19 vaccines for these patients.
Keywords: COVID-19; COVID-19 vaccination; SARS-CoV-2; cancer patients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Saini K.S., Tagliamento M., Lambertini M., McNally R., Romano M., Leone M., Curigliano G., de Azambuja E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer. 2020;139:43–50. doi: 10.1016/j.ejca.2020.08.011. - DOI - PMC - PubMed
-
- Lee L.Y.W., Cazier J.-B., Angelis V., Arnold R., Bisht V., Campton N.A., Chackathayil J., Cheng V.W.T., Curley H.M., Fittall M.W., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. - DOI - PMC - PubMed
-
- Pinato D.J., Zambelli A., Aguilar-Company J., Bower M., Sng C.C.T., Salazar R., Bertuzzi A., Brunet J., Mesia R., Seguí E., et al. Clinical Portrait of the SARS-CoV-2 Epidemic in European Patients with Cancer. Cancer Discov. 2020;10:1465–1474. doi: 10.1158/2159-8290.CD-20-0773. - DOI - PMC - PubMed
-
- El Sahly H.M., Baden L.R., Essink B., Doblecki-Lewis S., Martin J.M., Anderson E.J., Campbell T.B., Clark J., Jackson L.A., Fichtenbaum C.J., et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021;385:1774–1785. doi: 10.1056/NEJMoa2113017. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

