Refining the Role of Pyruvate Dehydrogenase Kinases in Glioblastoma Development
- PMID: 35954433
- PMCID: PMC9367285
- DOI: 10.3390/cancers14153769
Refining the Role of Pyruvate Dehydrogenase Kinases in Glioblastoma Development
Abstract
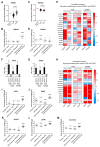
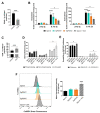
Glioblastoma (GB) are the most frequent brain cancers. Aggressive growth and limited treatment options induce a median survival of 12-15 months. In addition to highly proliferative and invasive properties, GB cells show cancer-associated metabolic characteristics such as increased aerobic glycolysis. Pyruvate dehydrogenase (PDH) is a key enzyme complex at the crossroads between lactic fermentation and oxidative pathways, finely regulated by PDH kinases (PDHKs). PDHKs are often overexpressed in cancer cells to facilitate high glycolytic flux. We hypothesized that targeting PDHKs, by disturbing cancer metabolic homeostasis, would alter GB progression and render cells vulnerable to additional cancer treatment. Using patient databases, distinct expression patterns of PDHK1 and PDHK2 in GB tissues were obvious. To disturb protumoral glycolysis, we modulated PDH activity through the genetic or pharmacological inhibition of PDHK in patient-derived stem-like spheroids. Striking effects of PDHKs inhibition using dichloroacetate were observed in vitro on cell morphology and metabolism, resulting in increased intracellular ROS levels and decreased proliferation and invasion. In vivo findings confirmed a reduction in tumor size and better survival of mice implanted with PDHK1 and PDHK2 knockout cells. Adding a radiotherapeutic protocol further resulted in a reduction in tumor size and improved mouse survival in our model.
Keywords: DCA; glioblastoma; invasion; lactate; pyruvate dehydrogenase kinases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

