Cancer-Associated Fibroblasts in a 3D Engineered Tissue Model Induce Tumor-like Matrix Stiffening and EMT Transition
- PMID: 35954473
- PMCID: PMC9367573
- DOI: 10.3390/cancers14153810
Cancer-Associated Fibroblasts in a 3D Engineered Tissue Model Induce Tumor-like Matrix Stiffening and EMT Transition
Abstract
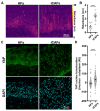
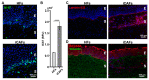
A tumor microenvironment is characterized by its altered mechanical properties. However, most models remain unable to faithfully recreate the mechanical properties of a tumor. Engineered models based on the self-assembly method have the potential to better recapitulate the stroma architecture and composition. Here, we used the self-assembly method based on a bladder tissue model to engineer a tumor-like environment. The tissue-engineered tumor models were reconstituted from stroma-derived healthy primary fibroblasts (HFs) induced into cancer-associated fibroblast cells (iCAFs) along with an urothelium overlay. The iCAFs-derived extracellular matrix (ECM) composition was found to be stiffer, with increased ECM deposition and remodeling. The urothelial cells overlaid on the iCAFs-derived ECM were more contractile, as measured by quantitative polarization microscopy, and displayed increased YAP nuclear translocation. We further showed that the proliferation and expression of epithelial-to-mesenchymal transition (EMT) marker in the urothelial cells correlate with the increased stiffness of the iCAFs-derived ECM. Our data showed an increased expression of EMT markers within the urothelium on the iCAFs-derived ECM. Together, our results demonstrate that our tissue-engineered tumor model can achieve stiffness levels comparable to that of a bladder tumor, while triggering a tumor-like response from the urothelium.
Keywords: 3D tumor models; ECM remodeling; EMT; bladder cancer; cancer-associated fibroblasts; cell contractility; engineered tumor microenvironment; matrix stiffness; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

