An Examination of the Anti-Cancer Properties of Plant Cannabinoids in Preclinical Models of Mesothelioma
- PMID: 35954477
- PMCID: PMC9367527
- DOI: 10.3390/cancers14153813
An Examination of the Anti-Cancer Properties of Plant Cannabinoids in Preclinical Models of Mesothelioma
Abstract
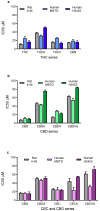
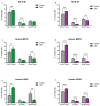
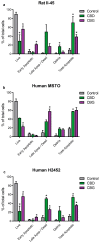
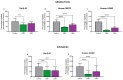
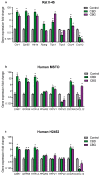
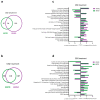
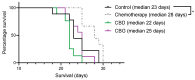
Mesothelioma is an aggressive cancer with limited treatment options and a poor prognosis. Phytocannabinoids possess anti-tumour and palliative properties in multiple cancers, however their effects in mesothelioma are unknown. We investigated the anti-cancer effects and potential mechanisms of action for several phytocannabinoids in mesothelioma cell lines. A panel of 13 phytocannabinoids inhibited growth of human (MSTO and H2452) and rat (II-45) mesothelioma cells in vitro, and cannabidiol (CBD) and cannabigerol (CBG) were the most potent compounds. Treatment with CBD or CBG resulted in G0/G1 arrest, delayed entry into S phase and induced apoptosis. CBD and CBG also significantly reduced mesothelioma cell migration and invasion. These effects were supported by changes in the expression of genes associated with the cell cycle, proliferation, and cell movement following CBD or CBG treatment. Gene expression levels of CNR1, GPR55, and 5HT1A also increased with CBD or CBG treatment. However, treatment with CBD or CBG in a syngeneic orthotopic rat mesothelioma model was unable to increase survival. Our data show that cannabinoids have anti-cancer effects on mesothelioma cells in vitro and alternatives of drug delivery may be needed to enhance their effects in vivo.
Keywords: anti-proliferative; apoptosis; cannabidiol; cannabigerol; cannabinoids; mesothelioma.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. I.S.M. and J.C.A. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded centre for medicinal cannabis research at the University of Sydney. J.C.A. and I.S.M. receive research funding from the Australian National Health and Medical Re-search Council (NHMRC) (GNT1161571). J.C.A. and I.S.M. have served as expert witnesses in various medicolegal cases involving cannabis and the cannabinoids. J.C.A. has received consulting fees from Creo Inc. and Medicinal Cannabis Industry Australia (MCIA). I.S.M. acts as a consultant to Kinoxis Therapeutics and the MCIA, and has received honoraria from Janssen. J.C.A., A.L.H., E.K.C., L.L.A. V.M.H. and I.S.M. hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554).
Figures
References
-
- Odgerel C.O., Takahashi K., Sorahan T., Driscoll T., Fitzmaurice C., Yoko O.M., Sawanyawisuth K., Furuya S., Tanaka F., Horie S., et al. Estimation of the global burden of mesothelioma deaths from incomplete national mortality data. Occup. Environ. Med. 2017;74:851–858. doi: 10.1136/oemed-2017-104298. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

