Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics
- PMID: 35954723
- PMCID: PMC9368073
- DOI: 10.3390/ijerph19159362
Innovative In Vitro Strategy for Assessing Aluminum Bioavailability in Oral Care Cosmetics
Abstract
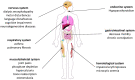
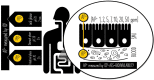
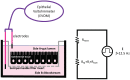
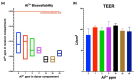
Aluminum is an element found in nature and in cosmetic products. It can interfere with the metabolism of other cations, thus inducing gastrointestinal disorder. In cosmetics, aluminum is used in antiperspirants, lipsticks, and toothpastes. The aim of this work is to investigate aluminum bioavailability after accidental oral ingestion derived from the use of a toothpaste containing a greater amount of aluminum hydroxide than advised by the Scientific Committee on Consumer Safety (SCCS). To simulate in vitro toothpaste accidental ingestion, the INFOGEST model was employed, and the amount of aluminum was measured through the ICP-AES analysis. Tissue barrier integrity was analyzed by measuring transepithelial electric resistance, and the tissue architecture was checked through light microscopy. The margin of safety was also calculated. Overall, our results indicate that the acute exposure to aluminum accidentally ingested from toothpastes is safe for the final user, even in amounts higher than SCCS indications.
Keywords: aluminum; bioavailability; margin of safety; next-generation risk assessment; oral care cosmetics.
Conflict of interest statement
The experiments were partially funded by Ludovico Martelli s.r.l. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Gad S. In: Aluminum. Wexler P., editor. Oxford University Press; Oxford, UK: 2014.
-
- Liang J., Liang X., Cao P., Wang X., Gao P., Ma N., Li N., Xu H. A Preliminary Investigation of Naturally Occurring Aluminum in Grains, Vegetables, and Fruits from Some Areas of China and Dietary Intake Assessment: Aluminum in Grains, Vegetables, and Fruits. J. Food Sci. 2019;84:701–710. doi: 10.1111/1750-3841.14459. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

