Factors Associated with the Quality and Transparency of National Guidelines: A Mixed-Methods Study
- PMID: 35954872
- PMCID: PMC9367745
- DOI: 10.3390/ijerph19159515
Factors Associated with the Quality and Transparency of National Guidelines: A Mixed-Methods Study
Abstract
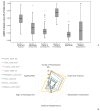
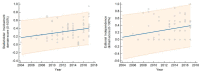
We assessed the methodological quality and transparency of all the national clinical practice guidelines that were published in Croatia up until 2017 and explored the factors associated with their quality rating. An in-depth quantitative and qualitative analysis was performed using rigorous methodology. We evaluated the guidelines using a validated AGREE II instrument with four raters; we used multiple linear regressions to identify the predictors of quality; and two focus groups, including guideline developers, to further explore the guideline development process. The majority of the guidelines (N = 74) were developed by medical societies. The guidelines' quality was rated low: the median standardized AGREE II score was low, 36% (IQR 28-42), and so were the overall-assessments. The aspects of the guidelines that were rated best were the "clarity of presentation" and the "scope and purpose" (median ≥ 59%); however, the other four domains received very low scores (15-33%). Overall, the guideline quality did not improve over time. The guidelines that were developed by medical societies scored significantly worse than those developed by governmental, or unofficial working groups (12-43% per domain). In focus group discussions, inadequate methodology, a lack of implementation systems in place, a lack of awareness about editorial independence, and broader expertise/perspectives in working groups were identified as factors behind the low scores. The factors identified as affecting the quality of the national guidelines may help stakeholders who are developing interventions and education programs aimed at improving guideline quality worldwide.
Keywords: clinical practice guidelines; education; focus group; guideline development; knowledge; methodological quality; national guidelines; public health.
Conflict of interest statement
A.M. declares support from the Croatian Science Foundation, grant No. IP-2014-09-7672 (“Professionalism in Health”), and Z.K. declares he is the president of CMA (pro bono professional service) and provided the study materials free of charge. Other authors declare no competing interest.
Figures

References
-
- IoM (IOM) In: Clinical Practice Guidelines We Can Trust. Graham R., Mancher M., Wolman D.M., Greenfield S., Steinberg E., editors. The National Academies Press (US); Washington, DC, USA: 2011. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

