Oxidation-Induced and Hydrothermal-Assisted Template-Free Synthesis of Mesoporous CeO2 for Adsorption of Acid Orange 7
- PMID: 35955142
- PMCID: PMC9369802
- DOI: 10.3390/ma15155209
Oxidation-Induced and Hydrothermal-Assisted Template-Free Synthesis of Mesoporous CeO2 for Adsorption of Acid Orange 7
Abstract
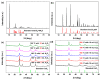
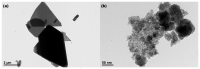
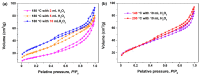
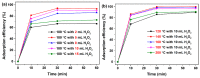
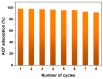
Hydrogen peroxide (H2O2), an accessible and eco-friendly oxidant, was employed for the template-free hydrothermal synthesis of mesoporous CeO2 based on a cerium carbonate precursor (Ce2(CO3)3•xH2O). Its microstructure and physicochemical properties were characterized by XRD, TEM and N2 sorption techniques. The formation of the CeO2 phase with a porous structure was strongly dependent on the presence of H2O2, while the values of the BET surface area, pore diameter and pore volume of CeO2 were generally related to the amount of H2O2 in the template-free hydrothermal synthesis. The BET surface area and pore volume of the mesoporous CeO2 synthesized hydrothermally at 180 °C with 10 mL H2O2 were 112.8 m2/g and 0.1436 cm3/g, respectively. The adsorption process had basically finished within 30 min, and the maximum adsorption efficiency within 30 min was 99.8% for the mesoporous CeO2 synthesized hydrothermally at 140 °C with 10 mL, when the initial AO7 concentration was 120 mg/L without pH preadjustment. The experimental data of AO7 adsorption were analyzed using the Langmuir and Freundlich isotherm modes. Moreover, the mesoporous CeO2 synthesized at 140 °C with 10 mL H2O2 was regenerated in successive adsorption-desorption cycles eight times without significant loss in adsorption capacity, suggesting that the as-synthesized mesoporous CeO2 in this work was suitable as an adsorbent for the efficient adsorption of AO7 dye from an aqueous solution.
Keywords: CeO2; adsorption; azo dye; hydrothermal; mesoporous; template-free.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Freeman H.S., Reife A. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2003. Dyes, Environmental Chemistry. - DOI
-
- Leulescu M., Rotaru A., Moanţă A., Iacobescu G., Pălărie I., Cioateră N., Popescu M., Criveanu M.C., Morîntale E., Bojan M., et al. Azorubine: Physical, thermal and bioactive properties of the widely employed food, pharmaceutical and cosmetic red azo dye material. J. Therm. Anal. Calorim. 2021;143:3945–3967. doi: 10.1007/s10973-021-10618-4. - DOI
-
- Ding Z., Li S., Zhou Y., Chen Z., Zhang F., Wu P., Ma W. LiBH4 for hydrogen storage: New perspectives. Nano Mater. Sci. 2020;2:109–119. doi: 10.1016/j.nanoms.2019.09.003. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

