Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review
- PMID: 35955386
- PMCID: PMC9369759
- DOI: 10.3390/ma15155450
Color Conversion Light-Emitting Diodes Based on Carbon Dots: A Review
Abstract
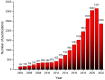
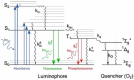
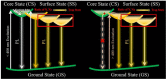
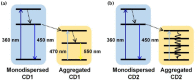
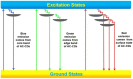
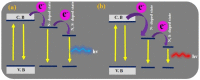
This paper reviews the state-of-the-art technologies, characterizations, materials (precursors and encapsulants), and challenges concerning multicolor and white light-emitting diodes (LEDs) based on carbon dots (CDs) as color converters. Herein, CDs are exploited to achieve emission in LEDs at wavelengths longer than the pump wavelength. White LEDs are typically obtained by pumping broad band visible-emitting CDs by an UV LED, or yellow-green-emitting CDs by a blue LED. The most important methods used to produce CDs, top-down and bottom-up, are described in detail, together with the process that allows one to embed the synthetized CDs on the surface of the pumping LEDs. Experimental results show that CDs are very promising ecofriendly candidates with the potential to replace phosphors in traditional color conversion LEDs. The future for these devices is bright, but several goals must still be achieved to reach full maturity.
Keywords: LEDs; carbon dots; carbon-dot-based light-emitting diodes; color conversion; multicolor light-emitting diodes; organic materials; phosphors; white light-emitting diodes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Jamaludin N., Rashid S.A., Tan T. Synthesis, Technology and Applications of Carbon Nanomaterials. Elsevier; Amsterdam, The Netherlands: 2019. Chapter 5—Natural biomass as carbon sources for the synthesis of photoluminescent carbon dots; pp. 109–134.
Publication types
LinkOut - more resources
Full Text Sources

