Deep Molecular Characterization of Milder Spinal Muscular Atrophy Patients Carrying the c.859G>C Variant in SMN2
- PMID: 35955418
- PMCID: PMC9368089
- DOI: 10.3390/ijms23158289
Deep Molecular Characterization of Milder Spinal Muscular Atrophy Patients Carrying the c.859G>C Variant in SMN2
Abstract
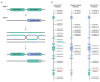
Spinal muscular atrophy (SMA) is a severe neuromuscular disorder caused by biallelic loss or pathogenic variants in the SMN1 gene. Copy number and modifier intragenic variants in SMN2, an almost identical paralog gene of SMN1, are known to influence the amount of complete SMN proteins. Therefore, SMN2 is considered the main phenotypic modifier of SMA, although genotype−phenotype correlation is not absolute. We present eleven unrelated SMA patients with milder phenotypes carrying the c.859G>C-positive modifier variant in SMN2. All were studied by a specific NGS method to allow a deep characterization of the entire SMN region. Analysis of two homozygous cases for the variant allowed us to identify a specific haplotype, Smn2-859C.1, in association with c.859G>C. Two other cases with the c.859G>C variant in their two SMN2 copies showed a second haplotype, Smn2-859C.2, in cis with Smn2-859C.1, assembling a more complex allele. We also identified a previously unreported variant in intron 2a exclusively linked to the Smn2-859C.1 haplotype (c.154-1141G>A), further suggesting that this region has been ancestrally conserved. The deep molecular characterization of SMN2 in our cohort highlights the importance of testing c.859G>C, as well as accurately assessing the SMN2 region in SMA patients to gain insight into the complex genotype−phenotype correlations and improve prognostic outcomes.
Keywords: SMN2 copies; next-generation sequencing; phenotype–genotype correlations; positive modifiers; spinal muscular atrophy.
Conflict of interest statement
The authors declare no conflict of interest in relation to this work.
Figures
References
-
- Sugarman E.A., Nagan N., Zhu H., Akmaev V.R., Zhou Z., Rohlfs E.M., Flynn K., Hendrickson B.C., Scholl T., Sirko-Osadsa D.A., et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: Clinical laboratory analysis of >72 400 specimens. Eur. J. Hum. Genet. 2012;20:27–32. doi: 10.1038/ejhg.2011.134. - DOI - PMC - PubMed

