Roles of RNA Sensors in Host Innate Response to Influenza Virus and Coronavirus Infections
- PMID: 35955436
- PMCID: PMC9368391
- DOI: 10.3390/ijms23158285
Roles of RNA Sensors in Host Innate Response to Influenza Virus and Coronavirus Infections
Abstract
Influenza virus and coronavirus are two important respiratory viruses, which often cause serious respiratory diseases in humans and animals after infection. In recent years, highly pathogenic avian influenza virus (HPAIV) and SARS-CoV-2 have become major pathogens causing respiratory diseases in humans. Thus, an in-depth understanding of the relationship between viral infection and host innate immunity is particularly important to the stipulation of effective control strategies. As the first line of defense against pathogens infection, innate immunity not only acts as a natural physiological barrier, but also eliminates pathogens through the production of interferon (IFN), the formation of inflammasomes, and the production of pro-inflammatory cytokines. In this process, the recognition of viral pathogen-associated molecular patterns (PAMPs) by host pattern recognition receptors (PRRs) is the initiation and the most important part of the innate immune response. In this review, we summarize the roles of RNA sensors in the host innate immune response to influenza virus and coronavirus infections in different species, with a particular focus on innate immune recognition of viral nucleic acids in host cells, which will help to develop an effective strategy for the control of respiratory infectious diseases.
Keywords: RNA sensors; coronavirus; influenza virus; innate immune response; viral RNA.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role.
Figures
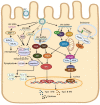
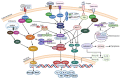
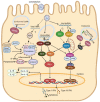
Similar articles
-
Human Nasal and Lung Tissues Infected Ex Vivo with SARS-CoV-2 Provide Insights into Differential Tissue-Specific and Virus-Specific Innate Immune Responses in the Upper and Lower Respiratory Tract.J Virol. 2021 Jun 24;95(14):e0013021. doi: 10.1128/JVI.00130-21. Epub 2021 Jun 24. J Virol. 2021. PMID: 33893170 Free PMC article.
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
-
Detection of Viral Infections by Innate Immunity.Biochem Pharmacol. 2021 Jan;183:114316. doi: 10.1016/j.bcp.2020.114316. Epub 2020 Nov 3. Biochem Pharmacol. 2021. PMID: 33152343 Review.
-
Recognition of Viral RNA by Pattern Recognition Receptors in the Induction of Innate Immunity and Excessive Inflammation During Respiratory Viral Infections.Viral Immunol. 2017 Jul/Aug;30(6):408-420. doi: 10.1089/vim.2016.0178. Epub 2017 Jun 13. Viral Immunol. 2017. PMID: 28609250 Review.
-
RNA sensors of the innate immune system and their detection of pathogens.IUBMB Life. 2017 May;69(5):297-304. doi: 10.1002/iub.1625. Epub 2017 Apr 4. IUBMB Life. 2017. PMID: 28374903 Free PMC article. Review.
Cited by
-
Hyper-Interferon Sensitive Influenza Induces Adaptive Immune Responses and Overcomes Resistance to Anti-PD-1 in Murine Non-Small Cell Lung Cancer.Cancer Immunol Res. 2024 Dec 3;12(12):1765-1779. doi: 10.1158/2326-6066.CIR-23-1075. Cancer Immunol Res. 2024. PMID: 39325056 Free PMC article.
-
Modified mRNA as a Treatment for Myocardial Infarction.Int J Mol Sci. 2023 Mar 1;24(5):4737. doi: 10.3390/ijms24054737. Int J Mol Sci. 2023. PMID: 36902165 Free PMC article. Review.
-
Viperin mutation is linked to immunity, immune cell dynamics, and metabolic alteration during VHSV infection in zebrafish.Front Immunol. 2023 Dec 19;14:1327749. doi: 10.3389/fimmu.2023.1327749. eCollection 2023. Front Immunol. 2023. PMID: 38173722 Free PMC article.
-
Differential Type-I Interferon Response in Buffy Coat Transcriptome of Individuals Infected with SARS-CoV-2 Gamma and Delta Variants.Int J Mol Sci. 2023 Aug 24;24(17):13146. doi: 10.3390/ijms241713146. Int J Mol Sci. 2023. PMID: 37685953 Free PMC article.
-
Next-Generation Vaccines: Nanovaccines in the Fight against SARS-CoV-2 Virus and beyond SARS-CoV-2.Biomed Res Int. 2023 May 4;2023:4588659. doi: 10.1155/2023/4588659. eCollection 2023. Biomed Res Int. 2023. PMID: 37181817 Free PMC article. Review.
References
-
- Chuang Y.C., Tseng J.C., Yang J.X., Liu Y.L., Yeh D.W., Lai C.Y., Yu G.Y., Hsu L.C., Huang C.M., Chuang T.H. Toll-Like Receptor 21 of Chicken and Duck Recognize a Broad Array of Immunostimulatory CpG-oligodeoxynucleotide Sequences. Vaccines. 2020;8:639. doi: 10.3390/vaccines8040639. - DOI - PMC - PubMed
-
- Ruemmele F.M., Beaulieu J.F., Lentze M.J. LPS-signaling in intestinal epithelial cells requires a functional Toll-like receptor 4. Gastroenterology. 2002;122:A152.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

