Hypoxia Aggravates Inhibition of Alveolar Epithelial Na-Transport by Lipopolysaccharide-Stimulation of Alveolar Macrophages
- PMID: 35955448
- PMCID: PMC9368968
- DOI: 10.3390/ijms23158315
Hypoxia Aggravates Inhibition of Alveolar Epithelial Na-Transport by Lipopolysaccharide-Stimulation of Alveolar Macrophages
Abstract
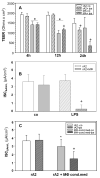
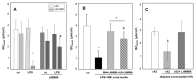
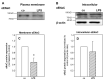
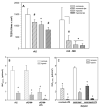
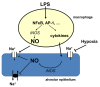
Inflammation and hypoxia impair alveolar barrier tightness, inhibit Na- and fluid reabsorption, and cause edema. We tested whether stimulated alveolar macrophages affect alveolar Na-transport and whether hypoxia aggravates the effects of inflammation, and tested for involved signaling pathways. Primary rat alveolar type II cells (rA2) were co-cultured with rat alveolar macrophages (NR8383) or treated with NR8383-conditioned media after stimulation with lipopolysaccharide (LPS; 1 µg/mL) and exposed to normoxia and hypoxia (1.5% O2). LPS caused a fast, transient increase in TNFα and IL-6 mRNA in macrophages and a sustained increase in inducible nitric oxide synthase (NOS2) mRNA in macrophages and in rA2 cells resulting in elevated nitrite levels and secretion of TNF-α and IL-6 into culture media. In normoxia, 24 h of LPS treated NR8383 decreased the transepithelial electrical resistance (TEER) of co-cultures, of amiloride-sensitive short circuit current (ISCΔamil); whereas Na/K-ATPase activity was not affected. Inhibition was also seen with conditioned media from LPS-stimulated NR8383 on rA2, but was less pronounced after dialysis to remove small molecules and nitrite. The effect of LPS-stimulated macrophages on TEER and Na-transport was fully prevented by the iNOS-inhibitor L-NMMA applied to co-cultures and to rA2 mono-cultures. Hypoxia in combination with LPS-stimulated NR8383 totally abolished TEER and ISCΔamil. These results indicate that the LPS-stimulation of alveolar macrophages impairs alveolar epithelial Na-transport by NO-dependent mechanisms, where part of the NO is produced by rA2 induced by signals from LPS stimulated alveolar macrophages.
Keywords: Na/K-ATPase; alveolar epithelial Na-channels; alveolar macrophages; hypoxia; iNOS; inflammation; pulmonary edema.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

