A Preclinical Study on Brugada Syndrome with a CACNB2 Variant Using Human Cardiomyocytes from Induced Pluripotent Stem Cells
- PMID: 35955449
- PMCID: PMC9368582
- DOI: 10.3390/ijms23158313
A Preclinical Study on Brugada Syndrome with a CACNB2 Variant Using Human Cardiomyocytes from Induced Pluripotent Stem Cells
Abstract
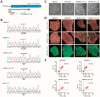
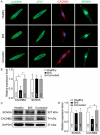
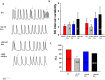
Aims: Some gene variants in the sodium channels, as well as calcium channels, have been associated with Brugada syndrome (BrS). However, the investigation of the human cellular phenotype and the use of drugs for BrS in presence of variant in the calcium channel subunit is still lacking. Objectives: The objective of this study was to establish a cellular model of BrS in the presence of a CACNB2 variant of uncertain significance (c.425C > T/p.S142F) using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) and test drug effects using this model. Methods and results: This study recruited cells from a patient with Brugada syndrome (BrS) and recurrent ventricular fibrillation carrying a missense variant in CACNB2 as well as from three healthy independent persons. These cells (hiPSC-CMs) generated from skin biopsies of healthy persons and the BrS patient (BrS-hiPSC-CMs) as well as CRISPR/Cas9 corrected cells (isogenic control, site-variant corrected) were used for this study. The hiPSC-CMs from the BrS patient showed a significantly reduced L-type calcium channel current (ICa-L) compared with the healthy control hiPSC-CMs. The inactivation curve was shifted to a more positive potential and the recovery from inactivation was accelerated. The protein expression of CACNB2 of the hiPSC-CMs from the BrS-patient was significantly decreased compared with healthy hiPSC-CMs. Moreover, the correction of the CACNB2 site-variant rescued the changes seen in the hiPSC-CMs of the BrS patient to the normal state. These data indicate that the CACNB2 gene variant led to loss-of-function of L-type calcium channels in hiPSC-CMs from the BrS patient. Strikingly, arrhythmia events were more frequently detected in BrS-hiPSC-CMs. Bisoprolol (beta-blockers) at low concentration and quinidine decreased arrhythmic events. Conclusions: The CACNB2 variant (c.425C > T/p.S142F) causes a loss-of-function of L-type calcium channels and is pathogenic for this type of BrS. Bisoprolol and quinidine may be effective for treating BrS with this variant.
Keywords: Brugada syndrome; CACNB gene; arrhythmias; human-induced pluripotent stem cell-derived cardiomyocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Milman A., Andorin A., Gourraud J.-B., Postema P.G., Sacher F., Mabo P., Kim S.-H., Juang J.J., Maeda S., Takahashi Y., et al. Profile of patients with Brugada syndrome presenting with their first documented arrhythmic event: Data from the Survey on Arrhythmic Events in BRUgada Syndrome (SABRUS) Heart Rhythm. 2018;15:716–724. doi: 10.1016/j.hrthm.2018.01.014. - DOI - PubMed
-
- Casado-Arroyo R., Berne P., Rao J.Y., Rodriguez-Mañero M., Levinstein M., Conte G., Sieira J., Namdar M., Ricciardi D., Chierchia G.B., et al. Long-Term Trends in Newly Diagnosed Brugada Syndrome: Implications for Risk Stratification. J. Am. Coll. Cardiol. 2016;68:614–623. doi: 10.1016/j.jacc.2016.05.073. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

