Platelet Lipidome Fingerprint: New Assistance to Characterize Platelet Dysfunction in Obesity
- PMID: 35955459
- PMCID: PMC9369067
- DOI: 10.3390/ijms23158326
Platelet Lipidome Fingerprint: New Assistance to Characterize Platelet Dysfunction in Obesity
Abstract
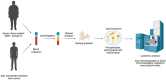
Obesity is associated with a pro-inflammatory and pro-thrombotic state that supports atherosclerosis progression and platelet hyper-reactivity. During the last decade, the platelet lipidome has been considered a treasure trove, as it is a source of biomarkers for preventing and treating different pathologies. The goal of the present study was to determine the lipid profile of platelets from non-diabetic, severely obese patients compared with their age- and sex-matched lean controls. Lipids from washed platelets were isolated and major phospholipids, sphingolipids and neutral lipids were analyzed either by gas chromatography or by liquid chromatography coupled to mass spectrometry. Despite a significant increase in obese patient's plasma triglycerides, there were no significant differences in the levels of triglycerides in platelets among the two groups. In contrast, total platelet cholesterol was significantly decreased in the obese group. The profiling of phospholipids showed that phosphatidylcholine and phosphatidylethanolamine contents were significantly reduced in platelets from obese patients. On the other hand, no significant differences were found in the sphingomyelin and ceramide levels, although there was also a tendency for reduced levels in the obese group. The outline of the glycerophospholipid and sphingolipid molecular species (fatty-acyl profiles) was similar in the two groups. In summary, these lipidomics data indicate that platelets from obese patients have a unique lipid fingerprint that may guide further studies and provide mechanistic-driven perspectives related to the hyperactivate state of platelets in obesity.
Keywords: lipidomics; obesity; platelets.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- WHO Consultation on Obesity & World Health Organization . Obesity: Preventing and Managing the Global Epidemic. Volume 894. World Health Organization; Geneva, Switzerland: 2000. pp. 1–253. Report of a WHO Consultation. - PubMed
-
- Badimon L., Hernández Vera R., Padró T., Vilahur G. Antithrombotic therapy in obesity. Thromb. Haemost. 2013;110:681–688. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

