Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A
- PMID: 35955466
- PMCID: PMC9368703
- DOI: 10.3390/ijms23158332
Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A
Abstract
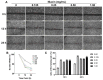
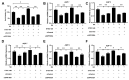
(1) Background: Changes in the expression of aquaporins (AQPs) in the intestine are proved to be associated with the attenuation of diarrhea. Diarrhea is a severe problem for postweaning piglets. Therefore, this study aimed to investigate whether niacin could alleviate diarrhea in weaned piglets by regulating AQPs expression and the underlying mechanisms; (2) Methods: 72 weaned piglets (Duroc × (Landrace × Yorkshire), 21 d old, 6.60 ± 0.05 kg) were randomly allotted into 3 groups for a 14-day feeding trial. Each treatment group included 6 replicate pens and each pen included 4 barrows (n = 24/treatment). Piglets were fed a basal diet (CON), a basal diet supplemented with 20.4 mg niacin/kg diet (NA) or the basal diet administered an antagonist for the GPR109A receptor (MPN). Additionally, an established porcine intestinal epithelial cell line (IPEC-J2) was used to investigate the protective effects and underlying mechanism of niacin on AQPs expression after Escherichia coli K88 (ETEC K88) treatment; (3) Results: Piglets fed niacin-supplemented diet had significantly decreased diarrhea rate, and increased mRNA and protein level of ZO-1, AQP 1 and AQP 3 in the colon compared with those administered a fed diet supplemented with an antagonist (p < 0.05). In addition, ETEC K88 treatment significantly reduced the cell viability, cell migration, and mRNA and protein expression of AQP1, AQP3, AQP7, AQP9, AQP11, and GPR109A in IPEC-J2 cells (p < 0.05). However, supplementation with niacin significantly prevented the ETEC K88-induced decline in the cell viability, cell migration, and the expression level of AQPs mRNA and protein in IPEC-J2 cells (p < 0.05). Furthermore, siRNA GPR109A knockdown significantly abrogated the protective effect of niacin on ETEC K88-induced cell damage (p < 0.05); (4) Conclusions: Niacin supplementation increased AQPs and ZO-1 expression to reduce diarrhea and intestinal damage through GPR109A pathway in weaned piglets.
Keywords: GPR109A; IPEC-J2 cell; aquaporins; diarrhea; niacin; piglets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


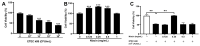


Similar articles
-
Differential expression of intestinal ion transporters and water channel aquaporins in young piglets challenged with enterotoxigenic Escherichia coli K88.J Anim Sci. 2017 Dec;95(12):5240-5252. doi: 10.2527/jas2017.1806. J Anim Sci. 2017. PMID: 29293799 Free PMC article.
-
Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with enterotoxigenic Escherichia coli K88.J Anim Sci. 2014 Apr;92(4):1496-503. doi: 10.2527/jas.2013-6619. Epub 2014 Feb 3. J Anim Sci. 2014. PMID: 24492550 Clinical Trial.
-
Tannic acid alleviates ETEC K88-induced intestinal damage through regulating the p62-keap1-Nrf2 and TLR4-NF-κB-NLRP3 pathway in IPEC-J2 cells.J Sci Food Agric. 2024 Jul;104(9):5186-5196. doi: 10.1002/jsfa.13343. Epub 2024 Feb 12. J Sci Food Agric. 2024. PMID: 38288747
-
Effects of dietary live yeast supplementation on growth performance, diarrhoea severity, intestinal permeability and immunological parameters of weaned piglets challenged with enterotoxigenic Escherichia coli K88.Br J Nutr. 2017 Dec;118(11):949-958. doi: 10.1017/S0007114517003051. Epub 2017 Nov 23. Br J Nutr. 2017. PMID: 29166952
-
Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets.Animals (Basel). 2021 Jul 23;11(8):2186. doi: 10.3390/ani11082186. Animals (Basel). 2021. PMID: 34438645 Free PMC article.
Cited by
-
Study on the effect of 3,5,6,7,8,3',4'-heptamethoxyflavone in Fructus Aurantii by regulating intestinal aquaporin in body fluids.Front Pharmacol. 2025 May 19;16:1544570. doi: 10.3389/fphar.2025.1544570. eCollection 2025. Front Pharmacol. 2025. PMID: 40458796 Free PMC article.
-
Nutrition strategies to control post-weaning diarrhea of piglets: From the perspective of feeds.Anim Nutr. 2024 Mar 26;17:297-311. doi: 10.1016/j.aninu.2024.03.006. eCollection 2024 Jun. Anim Nutr. 2024. PMID: 38800731 Free PMC article. Review.
-
The Effect of Vitamins on the Immune Systems of Pigs.Animals (Basel). 2024 Jul 21;14(14):2126. doi: 10.3390/ani14142126. Animals (Basel). 2024. PMID: 39061588 Free PMC article. Review.
-
Evaluation indicators of Traditional Chinese Medicine syndromes for gouty arthritis with damp heat accumulation and the effect of administering Tongfeng Qingxiao formula.J Tradit Chin Med. 2024 Dec;44(6):1204-1216. doi: 10.19852/j.cnki.jtcm.20240706.001. J Tradit Chin Med. 2024. PMID: 39617706 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

