Synovial Fluid Regulates the Gene Expression of a Pattern of microRNA via the NF-κB Pathway: An In Vitro Study on Human Osteoarthritic Chondrocytes
- PMID: 35955467
- PMCID: PMC9369022
- DOI: 10.3390/ijms23158334
Synovial Fluid Regulates the Gene Expression of a Pattern of microRNA via the NF-κB Pathway: An In Vitro Study on Human Osteoarthritic Chondrocytes
Abstract
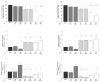
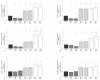
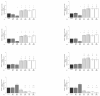
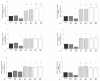
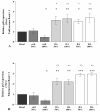
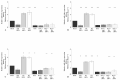
Synovial fluid (SF) represents the primary source of nutrients of articular cartilage and is implicated in maintaining cartilage metabolism. We investigated the effects of SF, from patients with osteoarthritis (OA), rheumatoid arthritis (RA), and controls, on a pattern of microRNA (miRNA) in human OA chondrocytes. Cells were stimulated with 50% or 100% SF for 24 h and 48 h. Apoptosis and superoxide anion production were detected by cytometry; miRNA (34a, 146a, 155, 181a), cytokines, metalloproteinases (MMPs), type II collagen (Col2a1), antioxidant enzymes, B-cell lymphoma (BCL)2, and nuclear factor (NF)-κB by real-time PCR. The implication of the NF-κB pathway was assessed by the use of NF-κB inhibitor (BAY-11-7082). RA and OA SF up-regulated miR-34a, -146a, -155, -181a, interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, MMP-1, MMP-13, and ADAMTs-5 gene expression, while it down-regulated Col2a1. Pathological SF also induced apoptosis, reduced viability, and decreased BCL2 mRNA, whereas it increased superoxide anions, the expression of antioxidant enzymes, p65 and p50 NF-κB. Opposite and positive results were obtained with 100% control SF. Pre-incubation with BAY-11-7082 counteracted SF effects on miRNA. We highlight the role of the SF microenvironment in regulating some miRNA involved in inflammation and cartilage degradation during OA and RA, via the NF-κB pathway.
Keywords: cartilage metabolism; chondrocytes; cytokines; inflammation; microRNA; osteoarthritis; rheumatoid arthritis; synovial fluid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
MicroRNA Mediate Visfatin and Resistin Induction of Oxidative Stress in Human Osteoarthritic Synovial Fibroblasts Via NF-κB Pathway.Int J Mol Sci. 2019 Oct 20;20(20):5200. doi: 10.3390/ijms20205200. Int J Mol Sci. 2019. PMID: 31635187 Free PMC article.
-
MicroRNA-34a and MicroRNA-181a Mediate Visfatin-Induced Apoptosis and Oxidative Stress via NF-κB Pathway in Human Osteoarthritic Chondrocytes.Cells. 2019 Aug 11;8(8):874. doi: 10.3390/cells8080874. Cells. 2019. PMID: 31405216 Free PMC article.
-
A Complex Relationship between Visfatin and Resistin and microRNA: An In Vitro Study on Human Chondrocyte Cultures.Int J Mol Sci. 2018 Dec 6;19(12):3909. doi: 10.3390/ijms19123909. Int J Mol Sci. 2018. PMID: 30563239 Free PMC article.
-
Inflammatory and Metabolic Signaling Interfaces of the Hypertrophic and Senescent Chondrocyte Phenotypes Associated with Osteoarthritis.Int J Mol Sci. 2023 Nov 17;24(22):16468. doi: 10.3390/ijms242216468. Int J Mol Sci. 2023. PMID: 38003658 Free PMC article. Review.
-
Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health.Int J Mol Sci. 2012;13(4):4202-4232. doi: 10.3390/ijms13044202. Epub 2012 Mar 30. Int J Mol Sci. 2012. PMID: 22605974 Free PMC article. Review.
Cited by
-
Modified Si Miao Powder granules alleviates osteoarthritis progression by regulating M1/M2 polarization of macrophage through NF-κB signaling pathway.Front Pharmacol. 2024 Jun 21;15:1361561. doi: 10.3389/fphar.2024.1361561. eCollection 2024. Front Pharmacol. 2024. PMID: 38974041 Free PMC article.
-
Role of lncRNA XIST/miR-146a Axis in Matrix Degradation and Apoptosis of Osteoarthritic Chondrocytes Through Regulation of MMP-13 and BCL2.Biology (Basel). 2025 Feb 20;14(3):221. doi: 10.3390/biology14030221. Biology (Basel). 2025. PMID: 40136478 Free PMC article.
-
MicroRNA as Possible Mediators of the Synergistic Effect of Celecoxib and Glucosamine Sulfate in Human Osteoarthritic Chondrocyte Exposed to IL-1β.Int J Mol Sci. 2023 Oct 8;24(19):14994. doi: 10.3390/ijms241914994. Int J Mol Sci. 2023. PMID: 37834442 Free PMC article.
-
Calebin A modulates inflammatory and autophagy signals for the prevention and treatment of osteoarthritis.Front Immunol. 2024 Mar 4;15:1363947. doi: 10.3389/fimmu.2024.1363947. eCollection 2024. Front Immunol. 2024. PMID: 38500879 Free PMC article.
-
Circulating serotonin and dopamine concentrations in osteoarthritis patients: a pilot study on the effect of pelotherapy.Int J Biometeorol. 2024 Jan;68(1):69-77. doi: 10.1007/s00484-023-02571-8. Epub 2023 Nov 14. Int J Biometeorol. 2024. PMID: 37962646 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

