Ciliary Neurotrophic Factor (CNTF) and Its Receptors Signal Regulate Cementoblasts Apoptosis through a Mechanism of ERK1/2 and Caspases Signaling
- PMID: 35955469
- PMCID: PMC9369201
- DOI: 10.3390/ijms23158335
Ciliary Neurotrophic Factor (CNTF) and Its Receptors Signal Regulate Cementoblasts Apoptosis through a Mechanism of ERK1/2 and Caspases Signaling
Abstract
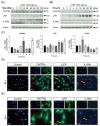
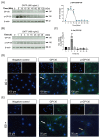
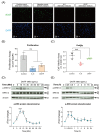
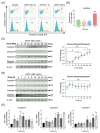
Ciliary neurotrophic factor (CNTF) was identified as a survival factor in various types of peripheral and central neurons, glia and non-neural cells. At present, there is no available data on the expression and localization of CNTF-receptors in cementoblasts as well as on the role of exogenous CNTF on this cell line. The purpose of this study was to determine if cementoblasts express CNTF-receptors and analyze the mechanism of its apoptotic regulation effects on cementoblasts. OCCM-30 cementoblasts were cultivated and stimulated kinetically using CNTF protein (NBP2-35168, Novus Biologicals). Quantified transcriptional (RT-qPCR) and translational (WB) products of CNTFRα, IL-6Rα (CD126), LIFR, p-GP130, GP130, p-ERK1/2, ERK1/2, Caspase-8, -9, -3 and cleaved-caspase-3 were evaluated. Immunofluorescence (IF) staining was applied to visualize the localization of the CNTF-receptors within cells. The apoptosis ratio was measured with an Annexin-V FITC/PI kit. The ERK1/2 antagonist (FR180204, Calbiochem) was added for further investigation by flow cytometry analysis. The CNTF-receptor complex (CNTFRα, LIFR, GP130) was functionally up-regulated in cementoblasts while cultivated with exogenous CNTF. CNTF significantly attenuated cell viability and proliferation for long-term stimulation. Flow cytometry analysis shows that CNTF enhanced the apoptosis after prolonged duration. However, after only a short-term period, CNTF halts the apoptosis of cementoblasts. Further studies revealed that CNTF activated phosphorylated GP130 and the anti-apoptotic molecule ERK1/2 signaling to participate in the regulation of the apoptosis ratio of cementoblasts. In conclusion, CNTF elicited the cellular functions through a notable induction of its receptor complex in cementoblasts. CNTF has an inhibitory effect on the cementoblast homeostasis. These data also elucidate a cellular mechanism for an exogenous CNTF-triggered apoptosis regulation in a mechanism of ERK1/2 and caspase signaling and provides insight into the complex cellular responses induced by CNTF in cementoblasts.
Keywords: CNTF; CNTF-receptors; ERK1/2; apoptosis; caspase; cementoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Schuster B., Kovaleva M., Sun Y., Regenhard P., Matthews V., Grotzinger J., Rose-John S., Kallen K.J. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J. Biol. Chem. 2003;278:9528–9535. doi: 10.1074/jbc.M210044200. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

