GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum
- PMID: 35955500
- PMCID: PMC9369126
- DOI: 10.3390/ijms23158355
GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum
Abstract
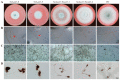
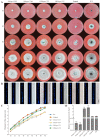
The Gtr1 protein was a member of the RagA subfamily of the Ras-like small GTPase superfamily and involved in phosphate acquisition, ribosome biogenesis and epigenetic control of gene expression in yeast. However, Gtr1 regulation sexual or asexual development in filamentous fungi is barely accepted. In the study, SeGtr1, identified from Stemphylium eturmiunum, could manipulate mycelial growth, nuclear distribution of mycelium and the morphology of conidia in Segtr1 silenced strains compared with its overexpression transformants, while the sexual activity of Segtr1 silenced strains were unchanged. SeASF1, a H3/H4 chaperone, participated in nucleosome assembly/disassembly, DNA replication and transcriptional regulation. Our experiments showed that deletion Seasf1 mutants produced the hyphal fusion and abnormal conidia. Notably, we characterized that Segtr1 was down-regulated in Se∆asf1 mutants and Seasf1 was also down-regulated in SiSegtr1 strains. We further confirmed that SeGtr1 interacted with SeASF1 or SeH4 in vivo and vitro, respectively. Thus, SeGtr1 can cooperate with SeASF1 to modulate asexual development in Stemphylium eturmiunum.
Keywords: ASF1; GTP binding protein Gtr1; Stemphylium eturmiunum; asexual development; interaction.
Conflict of interest statement
There are no conflict of interest for all authors and organizations that mentioned in this manuscript.
Figures

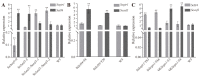


Similar articles
-
The Ddc1-Mec3-Rad17 sliding clamp regulates histone-histone chaperone interactions and DNA replication-coupled nucleosome assembly in budding yeast.J Biol Chem. 2014 Apr 11;289(15):10518-10529. doi: 10.1074/jbc.M114.552463. Epub 2014 Feb 25. J Biol Chem. 2014. PMID: 24573675 Free PMC article.
-
The intrinsic GTPase activity of the Gtr1 protein from Saccharomyces cerevisiae.BMC Biochem. 2012 Jun 24;13:11. doi: 10.1186/1471-2091-13-11. BMC Biochem. 2012. PMID: 22726655 Free PMC article.
-
Asf1, a loveseat for a histone couple.Cell. 2006 Nov 3;127(3):458-60. doi: 10.1016/j.cell.2006.10.021. Cell. 2006. PMID: 17081967
-
Dominant mutants of the Saccharomyces cerevisiae ASF1 histone chaperone bypass the need for CAF-1 in transcriptional silencing by altering histone and Sir protein recruitment.Genetics. 2006 Jun;173(2):599-610. doi: 10.1534/genetics.105.054783. Epub 2006 Apr 2. Genetics. 2006. PMID: 16582440 Free PMC article.
-
The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways.Chromosoma. 2007 Apr;116(2):79-93. doi: 10.1007/s00412-006-0087-z. Epub 2006 Dec 19. Chromosoma. 2007. PMID: 17180700 Review.
References
-
- Wu M.Y., Mead M.E., Lee M.K., Neuhaus G.F., Adpressa D.A., Martien J.I., Son Y.E., Moon H., Amador-Noguez D., Han K.H., et al. Transcriptomic, protein-DNA interaction, and metabolomic studies of VosA, VelB, and WetA in Aspergillus nidulans asexual spores. Mol. Biol. 2021;12:e03128-20. doi: 10.1128/mBio.03128-20. - DOI - PMC - PubMed
-
- Dyer P.S., Ingram D.S., Johnstone K. The control of sexual morphogenesis in the Ascomycotina. Biol. Rev. 1992;67:421–458. doi: 10.1111/j.1469-185X.1992.tb01189.x. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

