Molecular Mechanisms Underlying Intensive Care Unit-Acquired Weakness and Sarcopenia
- PMID: 35955530
- PMCID: PMC9368893
- DOI: 10.3390/ijms23158396
Molecular Mechanisms Underlying Intensive Care Unit-Acquired Weakness and Sarcopenia
Abstract
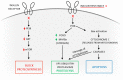
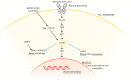
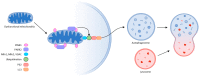
Skeletal muscle is a highly adaptable organ, and its amount declines under catabolic conditions such as critical illness. Aging is accompanied by a gradual loss of muscle, especially when physical activity decreases. Intensive care unit-acquired weakness is a common and highly serious neuromuscular complication in critically ill patients. It is a consequence of critical illness and is characterized by a systemic inflammatory response, leading to metabolic stress, that causes the development of multiple organ dysfunction. Muscle dysfunction is an important component of this syndrome, and the degree of catabolism corresponds to the severity of the condition. The population of critically ill is aging; thus, we face another negative effect-sarcopenia-the age-related decline of skeletal muscle mass and function. Low-grade inflammation gradually accumulates over time, inhibits proteosynthesis, worsens anabolic resistance, and increases insulin resistance. The cumulative consequence is a gradual decline in muscle recovery and muscle mass. The clinical manifestation for both of the above conditions is skeletal muscle weakness, with macromolecular damage, and a common mechanism-mitochondrial dysfunction. In this review, we compare the molecular mechanisms underlying the two types of muscle atrophy, and address questions regarding possible shared molecular mechanisms, and whether critical illness accelerates the aging process.
Keywords: intensive care unit-acquired weakness; muscle atrophy; proteostasis; rapamycin system; sarcopenia; ubiquitin–proteasome system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Intensive care unit-acquired weakness: unanswered questions and targets for future research.F1000Res. 2019 Apr 17;8:F1000 Faculty Rev-508. doi: 10.12688/f1000research.17376.1. eCollection 2019. F1000Res. 2019. PMID: 31069055 Free PMC article. Review.
-
Intensive Care Unit Acquired Weakness Is Associated with Rapid Changes to Skeletal Muscle Proteostasis.Cells. 2022 Dec 11;11(24):4005. doi: 10.3390/cells11244005. Cells. 2022. PMID: 36552769 Free PMC article.
-
Sarcopenia in critically ill patients.J Anesth. 2016 Oct;30(5):884-90. doi: 10.1007/s00540-016-2211-4. Epub 2016 Jul 4. J Anesth. 2016. PMID: 27376823 Review.
-
Critical illness neuromyopathy and muscle weakness in patients in the intensive care unit.AACN Adv Crit Care. 2009 Jul-Sep;20(3):243-53. doi: 10.1097/NCI.0b013e3181ac2551. AACN Adv Crit Care. 2009. PMID: 19638746 Review.
-
Unraveling the causes of sarcopenia: Roles of neuromuscular junction impairment and mitochondrial dysfunction.Physiol Rep. 2024 Jan;12(1):e15917. doi: 10.14814/phy2.15917. Physiol Rep. 2024. PMID: 38225199 Free PMC article. Review.
Cited by
-
Advancing critical care recovery: The pivotal role of machine learning in early detection of intensive care unit-acquired weakness.World J Clin Cases. 2024 Jul 26;12(21):4455-4459. doi: 10.12998/wjcc.v12.i21.4455. World J Clin Cases. 2024. PMID: 39070840 Free PMC article.
-
Effect of dimethyl fumarate on mitochondrial metabolism in a pediatric porcine model of asphyxia-induced in-hospital cardiac arrest.Sci Rep. 2024 Jun 15;14(1):13852. doi: 10.1038/s41598-024-64317-9. Sci Rep. 2024. PMID: 38879681 Free PMC article.
-
A Case Report of Probable Secondary Sarcopenia After Intensive Care Hospitalization.J Frailty Sarcopenia Falls. 2024 Jun 1;9(2):157-160. doi: 10.22540/JFSF-09-157. eCollection 2024 Jun. J Frailty Sarcopenia Falls. 2024. PMID: 38835624 Free PMC article.
-
Risk factors for ICU-acquired weakness in sepsis patients: A retrospective study of 264 patients.Heliyon. 2024 May 31;10(11):e32253. doi: 10.1016/j.heliyon.2024.e32253. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38867955 Free PMC article.
-
Role of GLP‑1 receptor agonists in sepsis and their therapeutic potential in sepsis‑induced muscle atrophy (Review).Int J Mol Med. 2025 May;55(5):74. doi: 10.3892/ijmm.2025.5515. Epub 2025 Mar 7. Int J Mol Med. 2025. PMID: 40052580 Free PMC article. Review.
References
-
- Hawkins R.B., Raymond S.L., Stortz J.A., Hiroyuki H., Brakenridge S.C., Gardner A., Efron P.A., Bihorac A., Segal M., Moore F.A., et al. Chronic critical illness and Persistent Inflammation, Immunosuppression and catabolism syndrome. Front. Immunol. 2018;9:1511. doi: 10.3389/fimmu.2018.01511. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

