Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light
- PMID: 35955538
- PMCID: PMC9369244
- DOI: 10.3390/ijms23158406
Removal of an Azo Dye from Wastewater through the Use of Two Technologies: Magnetic Cyclodextrin Polymers and Pulsed Light
Abstract
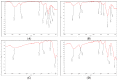
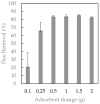
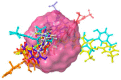
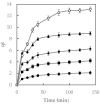
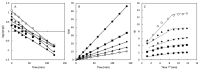
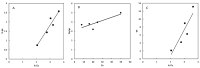
Water pollution by dyes is a huge environmental problem; there is a necessity to produce new decolorization methods that are effective, cost-attractive, and acceptable in industrial use. Magnetic cyclodextrin polymers offer the advantage of easy separation from the dye solution. In this work, the β-CD-EPI-magnetic (β-cyclodextrin-epichlorohydrin) polymer was synthesized, characterized, and tested for removal of the azo dye Direct Red 83:1 from water, and the fraction of non-adsorbed dye was degraded by an advanced oxidation process. The polymer was characterized in terms of the particle size distribution and surface morphology (FE-SEM), elemental analysis (EA), differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA), infrared spectrophotometry (IR), and X-ray powder diffraction (XRD). The reported results hint that 0.5 g and pH 5.0 were the best conditions to carry out both kinetic and isotherm models. A 30 min contact time was needed to reach equilibrium with a qmax of 32.0 mg/g. The results indicated that the pseudo-second-order and intraparticle diffusion models were involved in the assembly of Direct Red 83:1 onto the magnetic adsorbent. Regarding the isotherms discussed, the Freundlich model correctly reproduced the experimental data so that adsorption was confirmed to take place onto heterogeneous surfaces. The calculation of the thermodynamic parameters further demonstrates the spontaneous character of the adsorption phenomena (ΔG° = −27,556.9 J/mol) and endothermic phenomena (ΔH° = 8757.1 J/mol) at 25 °C. Furthermore, a good reusability of the polymer was evidenced after six cycles of regeneration, with a negligible decline in the adsorption extent (10%) regarding its initial capacity. Finally, the residual dye in solution after treatment with magnetic adsorbents was degraded by using an advanced oxidation process (AOP) with pulsed light and hydrogen peroxide (343 mg/L); >90% of the dye was degraded after receiving a fluence of 118 J/cm2; the discoloration followed a pseudo first-order kinetics where the degradation rate was 0.0196 cm2/J. The newly synthesized β-CD-EPI-magnetic polymer exhibited good adsorption properties and separability from water which, when complemented with a pulsed light-AOP, may offer a good alternative to remove dyes such as Direct Red 83:1 from water. It allows for the reuse of both the polymer and the dye in the dyeing process.
Keywords: adsorption kinetics; advanced oxidation process; organic contaminants; porous adsorbent; β-cyclodextrins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Characterization and adsorption of malachite green dye from aqueous solution onto Salix alba L. (Willow tree) leaves powder and its respective biochar.Int J Phytoremediation. 2023;25(5):646-657. doi: 10.1080/15226514.2022.2098909. Epub 2022 Jul 21. Int J Phytoremediation. 2023. PMID: 35862864
-
Adsorption Properties of β- and Hydroxypropyl-β-Cyclodextrins Cross-Linked with Epichlorohydrin in Aqueous Solution. A Sustainable Recycling Strategy in Textile Dyeing Process.Polymers (Basel). 2019 Feb 2;11(2):252. doi: 10.3390/polym11020252. Polymers (Basel). 2019. PMID: 30960236 Free PMC article.
-
Ultrafast and simultaneous removal of anionic and cationic dyes by nanodiamond/UiO-66 hybrid nanocomposite.Chemosphere. 2020 May;247:125882. doi: 10.1016/j.chemosphere.2020.125882. Epub 2020 Jan 16. Chemosphere. 2020. PMID: 32069713
-
A comprehensive review of anionic azo dyes adsorption on surface-functionalised silicas.Environ Sci Pollut Res Int. 2022 Nov;29(51):76565-76610. doi: 10.1007/s11356-022-23062-0. Epub 2022 Sep 27. Environ Sci Pollut Res Int. 2022. PMID: 36166120 Review.
-
Emerging adsorptive removal of azo dye by metal-organic frameworks.Chemosphere. 2016 Oct;160:30-44. doi: 10.1016/j.chemosphere.2016.06.065. Epub 2016 Jun 26. Chemosphere. 2016. PMID: 27355417 Review.
Cited by
-
Magnetic Luffa-Leaf-Derived Hierarchical Porous Biochar for Efficient Removal of Rhodamine B and Tetracycline Hydrochloride.Int J Mol Sci. 2022 Dec 11;23(24):15703. doi: 10.3390/ijms232415703. Int J Mol Sci. 2022. PMID: 36555345 Free PMC article.
-
Metal Oxide Hydrogel Composites for Remediation of Dye-Contaminated Wastewater: Principal Component Analysis.Gels. 2022 Oct 30;8(11):702. doi: 10.3390/gels8110702. Gels. 2022. PMID: 36354610 Free PMC article.
-
Biopolymer Composites 2022.Int J Mol Sci. 2023 Mar 29;24(7):6430. doi: 10.3390/ijms24076430. Int J Mol Sci. 2023. PMID: 37047403 Free PMC article.
-
Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control.Int J Mol Sci. 2022 Nov 15;23(22):14082. doi: 10.3390/ijms232214082. Int J Mol Sci. 2022. PMID: 36430558 Free PMC article.
-
Cow Dung-Based Biochar Materials Prepared via Mixed Base and Its Application in the Removal of Organic Pollutants.Int J Mol Sci. 2022 Sep 3;23(17):10094. doi: 10.3390/ijms231710094. Int J Mol Sci. 2022. PMID: 36077497 Free PMC article.
References
-
- Kyzas G.Z., Siafaka P.I., Pavlidou E.G., Chrissafis K.J., Bikiaris D.N. Synthesis and Adsorption Application of Succinyl-Grafted Chitosan for the Simultaneous Removal of Zinc and Cationic Dye from Binary Hazardous Mixtures. Chem. Eng. J. 2015;259:438–448. doi: 10.1016/j.cej.2014.08.019. - DOI
-
- Crini G. Non-Conventional Adsorbents for Dye Removal. In: Sharma S.K., editor. Green Chemistry for Dyes Removal from Wastewater. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2015. pp. 359–407. - DOI
-
- Vakili M., Rafatullah M., Salamatinia B., Abdullah A.Z., Ibrahim M.H., Tan K.B., Gholami Z., Amouzgar P. Application of Chitosan and Its Derivatives as Adsorbents for Dye Removal from Water and Wastewater: A Review. Carbohydr. Polym. 2014;113:115–130. doi: 10.1016/j.carbpol.2014.07.007. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

