Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry
- PMID: 35955568
- PMCID: PMC9369277
- DOI: 10.3390/ijms23158434
Increased Angiogenesis and Lymphangiogenesis in Adenomyosis Visualized by Multiplex Immunohistochemistry
Abstract
There is evidence for increased angiogenesis in the (ectopic) endometrium of adenomyosis patients under the influence of vascular endothelial growth factor (VEGF). VEGF stimulates both angiogenesis and lymph-angiogenesis. However, information on lymph vessels in the (ectopic) endometrium of adenomyosis patients is lacking. In this retrospective matched case-control study, multiplex immunohistochemistry was performed on thirty-eight paraffin embedded specimens from premenopausal women who had undergone a hysterectomy at the Amsterdam UMC between 2001 and 2018 to investigate the evidence for (lymph) angiogenesis in the (ectopic) endometrium or myometrium of patients with adenomyosis versus controls with unrelated pathologies. Baseline characteristics of both groups were comparable. In the proliferative phase, the blood and lymph vessel densities were, respectively, higher in the ectopic and eutopic endometrium of patients with adenomyosis than in the endometrium of controls. The relative number of blood vessels without α-smooth muscle actinin (α SMA) was higher in the eutopic and ectopic endometrium of adenomyosis patients versus controls. The level of VEGF staining intensity was highest in the myometrium but did not differ between patients with adenomyosis or controls. The results indicate increased angiogenesis and lymphangiogenesis in the (ectopic) endometrium affected by adenomyosis. The clinical relevance of our findings should be confirmed in prospective clinical studies.
Keywords: adenomyosis; angiogenesis; ectopic endometrium; histology; lymphangiogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
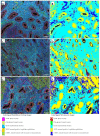


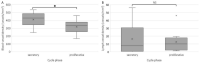
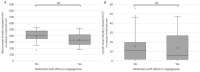
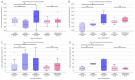



Similar articles
-
Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis.Hum Reprod. 2016 Apr;31(4):734-49. doi: 10.1093/humrep/dew018. Epub 2016 Feb 22. Hum Reprod. 2016. PMID: 26908845
-
Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review.Hum Reprod Update. 2019 Sep 11;25(5):647-671. doi: 10.1093/humupd/dmz024. Hum Reprod Update. 2019. PMID: 31504506 Free PMC article.
-
Matrix metalloproteinase-2 and -9 expression correlated with angiogenesis in human adenomyosis.Gynecol Obstet Invest. 2006;62(4):229-35. doi: 10.1159/000094426. Epub 2006 Jul 7. Gynecol Obstet Invest. 2006. PMID: 16837781
-
Expression of vascular endothelial growth factor (VEGF), hypoxia inducible factor-1alpha (HIF-1alpha), and microvessel density in endometrial tissue in women with adenomyosis.Int J Gynecol Pathol. 2009 Mar;28(2):157-63. doi: 10.1097/PGP.0b013e318182c2be. Int J Gynecol Pathol. 2009. PMID: 19188818
-
Structural and molecular features of the endomyometrium in endometriosis and adenomyosis.Hum Reprod Update. 2014 May-Jun;20(3):386-402. doi: 10.1093/humupd/dmt052. Epub 2013 Oct 18. Hum Reprod Update. 2014. PMID: 24140719 Review.
Cited by
-
Artificial intelligence-based tissue segmentation and cell identification in multiplex-stained histological endometriosis sections.Hum Reprod. 2025 Mar 1;40(3):450-460. doi: 10.1093/humrep/deae267. Hum Reprod. 2025. PMID: 39724530 Free PMC article.
-
Human Endometrial Pericytes: A Comprehensive Overview of Their Physiological Functions and Implications in Uterine Disorders.Cells. 2024 Sep 9;13(17):1510. doi: 10.3390/cells13171510. Cells. 2024. PMID: 39273080 Free PMC article. Review.
-
Endometrial Inflammation and Impaired Spontaneous Decidualization: Insights into the Pathogenesis of Adenomyosis.Int J Environ Res Public Health. 2023 Feb 20;20(4):3762. doi: 10.3390/ijerph20043762. Int J Environ Res Public Health. 2023. PMID: 36834456 Free PMC article. Review.
-
Anti-angiogenic therapy as potential treatment for adenomyosis.Angiogenesis. 2025 Jan 25;28(1):12. doi: 10.1007/s10456-024-09960-6. Angiogenesis. 2025. PMID: 39862328 Free PMC article.
-
Comprehensive transcriptional atlas of human adenomyosis deciphered by the integration of single-cell RNA-sequencing and spatial transcriptomics.Protein Cell. 2024 Jul 1;15(7):530-546. doi: 10.1093/procel/pwae012. Protein Cell. 2024. PMID: 38486356 Free PMC article.
References
-
- Griffioen A.W., Molema G. Angiogenesis: Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 2000;52:237–268. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

