Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ
- PMID: 35955585
- PMCID: PMC9368839
- DOI: 10.3390/ijms23158450
Unravelling the Inflammatory Processes in the Early Stages of Diabetic Nephropathy and the Potential Effect of (Ss)-DS-ONJ
Abstract
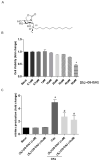
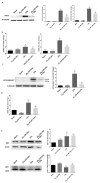
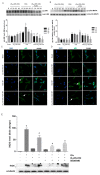
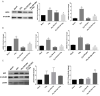
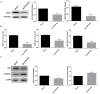
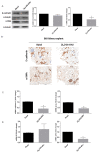
Inflammatory processes play a central role in the pathogenesis of diabetic nephropathy (DN) in the early stages of the disease. The authors demonstrate that the glycolipid mimetic (Ss)-DS-ONJ is able to abolish inflammation via the induction of autophagy flux and provokes the inhibition of inflammasome complex in ex vivo and in vitro models, using adult kidney explants from BB rats. The contribution of (Ss)-DS-ONJ to reducing inflammatory events is mediated by the inhibition of classical stress kinase pathways and the blocking of inflammasome complex activation. The (Ss)-DS-ONJ treatment is able to inhibit the epithelial-to-mesenchymal transition (EMT) progression, but only when the IL18 levels are reduced by the treatment. These findings suggest that (Ss)-DS-ONJ could be a novel, and multifactorial treatment for DN.
Keywords: (Ss)-DS-ONJ; autophagy; diabetic nephropathy; epithelial mesenchymal transition; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

