Key Residues Affecting Binding Affinity of Sirex noctilio Fabricius Odorant-Binding Protein (SnocOBP9) to Aggregation Pheromone
- PMID: 35955589
- PMCID: PMC9369295
- DOI: 10.3390/ijms23158456
Key Residues Affecting Binding Affinity of Sirex noctilio Fabricius Odorant-Binding Protein (SnocOBP9) to Aggregation Pheromone
Abstract
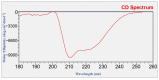
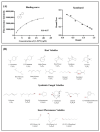
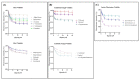
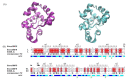
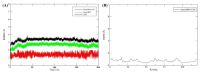
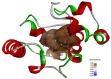
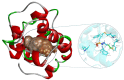
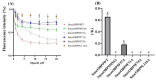
Sirex noctilio Fabricius (Hymenoptera Siricidae) is a major quarantine pest responsible for substantial economic losses in the pine industry. To achieve better pest control, (Z)-3-decen-ol was identified as the male pheromone and used as a field chemical trapping agent. However, the interactions between odorant-binding proteins (OBPs) and pheromones are poorly described. In this study, SnocOBP9 had a higher binding affinity with Z3D (Ki = 1.53 ± 0.09 μM) than other chemical ligands. Molecular dynamics simulation and binding mode analysis revealed that several nonpolar residues were the main drivers for hydrophobic interactions between SnocOBP9 and Z3D. Additionally, computational alanine scanning results indicated that five amino acids (MET54, PHE57, PHE71, PHE74, LEU116) in SnocOBP9 could potentially alter the binding affinity to Z3D. Finally, we used single-site-directed mutagenesis to substitute these five residues with alanine. These results imply that the five residues play crucial roles in the SnocOBP9-Z3D complex. Our research confirmed the function of SnocOBP9, uncovered the key residues involved in SnocOBP9-Z3D interactions, and provides an inspiration to improve the effects of pheromone agent traps.
Keywords: Sirex noctilio; aggregation pheromone; computational simulation; fluorescence binding assay; odorant-binding proteins; site-directed mutagenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Antennal transcriptome analysis of olfactory genes and characterizations of odorant binding proteins in two woodwasps, Sirex noctilio and Sirex nitobei (Hymenoptera: Siricidae).BMC Genomics. 2021 Mar 10;22(1):172. doi: 10.1186/s12864-021-07452-1. BMC Genomics. 2021. PMID: 33691636 Free PMC article.
-
Identification of odorant binding proteins in Carpomya vesuviana and their binding affinity to the male-borne semiochemicals and host plant volatiles.J Insect Physiol. 2017 Jul;100:100-107. doi: 10.1016/j.jinsphys.2017.05.013. Epub 2017 May 29. J Insect Physiol. 2017. PMID: 28571710
-
Key Amino Acid Residues Influencing Binding Affinities of Pheromone-Binding Protein from Athetis lepigone to Two Sex Pheromones.J Agric Food Chem. 2020 Jun 3;68(22):6092-6103. doi: 10.1021/acs.jafc.0c01572. Epub 2020 May 19. J Agric Food Chem. 2020. PMID: 32392414
-
Investigating SnocCSP4 expression and key compound interactions with SnocOBP4 in Sirex noctilio Fabricius (Hymenoptera: Siricidae).Int J Biol Macromol. 2023 Aug 30;247:125827. doi: 10.1016/j.ijbiomac.2023.125827. Epub 2023 Jul 13. Int J Biol Macromol. 2023. PMID: 37453637
-
The 40-Year Mystery of Insect Odorant-Binding Proteins.Biomolecules. 2021 Mar 30;11(4):509. doi: 10.3390/biom11040509. Biomolecules. 2021. PMID: 33808208 Free PMC article. Review.
References
-
- Skoczek A., Piesik D., Wenda-Piesik A., Buszewski B., Bocianowski J., Wawrzyniak M. Volatile organic compounds released by maize following herbivory or insect extract application and communication between plants. J. Appl. Entomol. 2017;141:630–643. doi: 10.1111/jen.12367. - DOI
-
- Piesik D., Rochat D., Delaney K., Marion-Poll F. Orientation of European corn borer first instar larvae to synthetic green leaf volatiles. J. Appl. Entomol. 2013;137:234–240. doi: 10.1111/j.1439-0418.2012.01719.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

