An Overview of NRF2-Activating Compounds Bearing α,β-Unsaturated Moiety and Their Antioxidant Effects
- PMID: 35955599
- PMCID: PMC9369284
- DOI: 10.3390/ijms23158466
An Overview of NRF2-Activating Compounds Bearing α,β-Unsaturated Moiety and Their Antioxidant Effects
Abstract
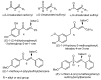
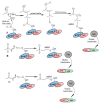
The surge of scientific interest in the discovery of Nuclear Factor Erythroid 2 (NFE2)-Related Factor 2 (NRF2)-activating molecules underscores the importance of NRF2 as a therapeutic target especially for oxidative stress. The chemical reactivity and biological activities of several bioactive compounds have been linked to the presence of α,β-unsaturated structural systems. The α,β-unsaturated carbonyl, sulfonyl and sulfinyl functional groups are reportedly the major α,β-unsaturated moieties involved in the activation of the NRF2 signaling pathway. The carbonyl, sulfonyl and sulfinyl groups are generally electron-withdrawing groups, and the presence of the α,β-unsaturated structure qualifies them as suitable electrophiles for Michael addition reaction with nucleophilic thiols of cysteine residues within the proximal negative regulator of NRF2, Kelch-like ECH-associated protein 1 (KEAP1). The physicochemical property such as good lipophilicity of these moieties is also an advantage because it ensures solubility and membrane permeability required for the activation of the cytosolic NRF2/KEAP1 system. This review provides an overview of the reaction mechanism of α,β-unsaturated moiety-bearing compounds with the NRF2/KEAP1 complex, their pharmacological properties, structural activity-relationship and their effect on antioxidant and anti-inflammatory responses. As the first of its kind, this review article offers collective and comprehensive information on NRF2-activators containing α,β-unsaturated moiety with the aim of broadening their therapeutic prospects in a wide range of oxidative stress-related diseases.
Keywords: KEAP1; NRF2; Parkinson’s disease; anti-inflammatory; antioxidant; carbonyl; sulfinyl; sulfonyl; α,β-unsaturated moiety.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
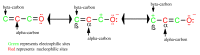


References
-
- Sulsen V.P., Martino V.S. Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects. 1st ed. Springer Nature; Cham, Switzerland: 2018.
-
- Carlstrom K.E., Chinthkindi P.K., Espinosa B., Nimer F.A., Arner E.S.J., Arvidsson P.I., Piehl F., Johnasson K. Characterization of more selective central nervous system NRF2-activating novel vinyl sulfoxime compounds compared to dimethyl fumarate. Neurotherapeutics. 2020;17:114–1152. doi: 10.1007/s13311-020-00855-0. - DOI - PMC - PubMed
-
- Shim S.Y., Hwang H.S., Nam G., Choi K. Synthesis and NRF2 activating ability of thiourea and vinyl sulfoxide derivatives. Bull. Korean Chem. Soc. 2013;34:2317–2320. doi: 10.5012/bkcs.2013.34.8.2317. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

